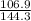You heat a 5.2 gram lead ball (specific heat capacity = 0.128 Joules/g-deg) to 183. ∘ C. You then drop the ball into 34.5 milliliters of water (density 1 g/ml; specific heat capacity = 4.184 J/g-deg ) at 22.4 ∘ C. What is the final temperature of the water when the lead and water reach thermal equilibrium?

Answers: 3


Another question on Physics

Physics, 21.06.2019 21:50
Which of the following is a homogenous mixture? o a. a toy box filled with toys o b. blood o c. trail mix o d. spaghetti and meatballs submit
Answers: 2

Physics, 22.06.2019 12:00
If the new moon happens on january 15th, what shape will it be on february 6th?
Answers: 1

Physics, 22.06.2019 14:10
The number of passengers who arrive at the platform of a subway station for the 10 am train is a random variable with a mean of 120 and a variance of 16. find the lower bound of the probability that there will be between 100 and 140 passengers (round off to second decimal place).
Answers: 3

Physics, 22.06.2019 16:30
Acalorimeter uses the principle that thermal energy flows from hotter material to colder material until both reach the same
Answers: 1
You know the right answer?
You heat a 5.2 gram lead ball (specific heat capacity = 0.128 Joules/g-deg) to 183. ∘ C. You then dr...
Questions




World Languages, 25.12.2019 02:31



Mathematics, 25.12.2019 02:31













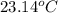
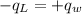 .............................1
.............................1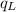 is the heat energy of the lead ball;
is the heat energy of the lead ball;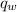 is the heat energy of water;
is the heat energy of water;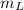 is the mass of the lead ball = 5.2 g
is the mass of the lead ball = 5.2 g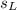 is the specific heat capacity of the lead ball = 0.128 Joules/g-deg
is the specific heat capacity of the lead ball = 0.128 Joules/g-deg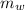 is the mass of water = volume x density = 34.5 ml x 1 g/ml = 34.5 g
is the mass of water = volume x density = 34.5 ml x 1 g/ml = 34.5 g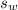 is the specific heat capacity of water = 4.184 J/g-deg
is the specific heat capacity of water = 4.184 J/g-deg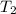 -22.4)
-22.4)