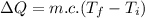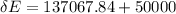Suppose you warm up 520 grams of water (about half a liter, or about a pint) on a stove, and while this is happening, you also stir the water with a beater, doing 5multiply. gif104 J of work on the water. After the large-scale motion of the water has dissipated away, the temperature of the water is observed to have risen from 21°C to 84°C.
What was the change in the thermal energy of the water?
deltacapEthermal =?
Taking the water as the system, how much energy transfer due to a temperature difference (microscopic work) Q was there across the system boundary?
Q = ?
Taking the water as the system, what was the energy change of the surroundings?
deltacapEsurroundings=?

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:00
Asubmarine has a "crush depth" (that is, the depth at which water pressure will crush the submarine) of 250 m. what is the approximate pressure (water plus atmospheric) at this depth? (recall that the density of seawater is 1025 kg/m3, g = 9.81 m/s2, and 1 kg/(ms2) = 1 pa = 9.8692 10-6 atm.) a. 34.8 atm b. 24.8 atm c. 25.8 atm d. 7.8 atm
Answers: 2


Physics, 22.06.2019 09:30
1. how to locate the image in converging(concave) mirror and diverging (convex) mirror with salt. 2. how to locate the image in a converging (convex) lens and diverging (concave) lens with salt.
Answers: 1

Physics, 22.06.2019 11:30
Considering only the earth's rotation, determine how much later the asteroid would have had to arrive to put the explosion above helsinki at longitude 25˚ e? this would have obliterated the city.
Answers: 1
You know the right answer?
Suppose you warm up 520 grams of water (about half a liter, or about a pint) on a stove, and while t...
Questions


Mathematics, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00

Social Studies, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00



Spanish, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00



Social Studies, 23.01.2021 01:00



Biology, 23.01.2021 01:00

History, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00


 is the change in the thermal energy of water this is also the amount of energy crossing the system boundary due to temperature difference.
is the change in the thermal energy of water this is also the amount of energy crossing the system boundary due to temperature difference.
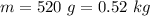 work done on the water by stirring,
work done on the water by stirring,  initial temperature of water,
initial temperature of water, 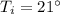 final temperature of water,
final temperature of water,