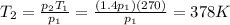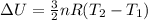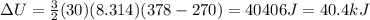A sealed tank contains 30 moles of an ideal gas at an initial temperature of 270 K. The pressure of the gas is increased until the final pressure equals 1.4 times the initial pressure. The heat capacity at constant pressure of the gas is 32.0 J(mol*K) What is the change in the internal energy of the gas? Let the ideal-gas constant R = 8.314 J/(mol • K).
130 kJ
77 kJ
-23 kJ
100 kJ
-50 kJ

Answers: 2


Another question on Physics


Physics, 22.06.2019 18:00
Which of the following is not a physical property of mattera. melting pointb.heat of combustionc. viscosityd. boiling point
Answers: 1

Physics, 22.06.2019 23:30
A6.0-kilogram cart initially traveling at 4.0 meters per second east accelerates uniformly at 0.50 meter per second squared east for 3.0 seconds. what is the speed of the cart at the end of this 3.0 second interval? (1) 1.5 m/s (3) 3.0 m/s (2) 5.5 m/s (4) 7.0 m/s
Answers: 1

Physics, 23.06.2019 00:30
What types of radiation make up the electromagnetic spectrum?
Answers: 2
You know the right answer?
A sealed tank contains 30 moles of an ideal gas at an initial temperature of 270 K. The pressure of...
Questions

Mathematics, 07.05.2021 20:50





Mathematics, 07.05.2021 20:50


English, 07.05.2021 20:50



Mathematics, 07.05.2021 20:50

English, 07.05.2021 20:50



Arts, 07.05.2021 20:50



Spanish, 07.05.2021 20:50


Mathematics, 07.05.2021 20:50

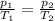
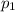 is the initial pressure of the gas
is the initial pressure of the gas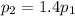 is the final pressure
is the final pressure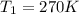 is the initial temperature
is the initial temperature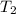 is the final temperature
is the final temperature