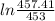Answers: 2


Another question on Physics

Physics, 21.06.2019 22:00
Bob camina 200 m sur, luego trota 400 m suroeste, luego camina 200 m en una dirección 30? norte del este. a. dibuje una gráfica de los movimientos de bob. use una regla y transportador. b. use método gráfico y analíitico para hallar el desplazamiento total que bob recorrió. (magnitud y dirección) c. compare los resultados obtenidos por el método gráfico y analítico. (por ciento de diferencia).
Answers: 2

Physics, 21.06.2019 23:20
From center station, a train departs every 30 minutes on the fast line and a train departs every 50 minutes on the state line. if two trains depart from center station at 8: 00 a.m., one on each of the two lines, what is the next time that two trains, one on each line, will depart at the same time?
Answers: 1

Physics, 22.06.2019 04:00
All the simple machines make work easier to do by changing the or of a force. a. size; type b. work; type c. size; direction d. type; direction
Answers: 2

Physics, 22.06.2019 09:00
Inside of a windmill or a dam is a that is used to transform one type of energy into electrical energy. a. motor b. generator c. transformer d. power pack
Answers: 2
You know the right answer?
A 25-kg iron block initially at 350oC is quenched in an insulated tank that contains 100 kg of water...
Questions

Chemistry, 29.04.2021 17:20

Mathematics, 29.04.2021 17:20


History, 29.04.2021 17:20


Mathematics, 29.04.2021 17:20




Geography, 29.04.2021 17:20


History, 29.04.2021 17:20



Mathematics, 29.04.2021 17:20

Physics, 29.04.2021 17:20


Health, 29.04.2021 17:20

History, 29.04.2021 17:20

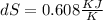
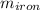 = 25 kg
= 25 kg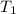 = 350°c = 623 K
= 350°c = 623 K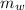 = 100 kg
= 100 kg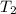 = 180°c = 453 K
= 180°c = 453 K 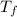
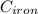 (
( 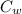 (
( 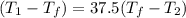
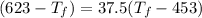
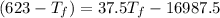
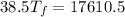


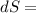 25 × 0.448 ×
25 × 0.448 × 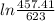 + 100 × 4.2 ×
+ 100 × 4.2 ×