
Physics, 19.03.2020 00:03 jalenshayewilliams
Acetylene gas (C2H2) is used in welding torches. When it reacts with oxygen, it produces carbon dioxide (CO2) and steam (H2O). The reaction can be described by the equation:
2C2H2 + 5O2 ➔ 4CO2 + 2H2O. How much mass of C2H2 is needed to react with 68.1 g of O2 to produce 75.0 g of CO2 and 15.35 g of steam?

Answers: 1


Another question on Physics

Physics, 22.06.2019 12:00
For the car in the picture ,in which direction is the normal force? -into the screen -down -out of the screen -up
Answers: 2


Physics, 22.06.2019 20:30
You are doing a science experiment with a fahrenheit thermometer. your data must be in degrees celsius. if you measure a temperature of 125°f, what is this temperature in degrees celsius
Answers: 1

Physics, 22.06.2019 22:10
7. see worksheet 1 for values of variables x1, x2 and x3 and answer the following questions: a. for each variable find the mean, median, coefficient of skewness, range and population standard deviation. b. compared to variable x1, how are the mean and median affected by extreme values (outliers) seen in x2 and x3. c. is the median or mean the better measure of location for x2 and x3? explain. d. explain the differences in the magnitudes of the skewness coefficients for the three variables. e. what is the relationship between the range and standard deviation looking across the three variables?
Answers: 1
You know the right answer?
Acetylene gas (C2H2) is used in welding torches. When it reacts with oxygen, it produces carbon diox...
Questions




Mathematics, 27.08.2020 07:01



Mathematics, 27.08.2020 07:01

Mathematics, 27.08.2020 07:01


Mathematics, 27.08.2020 07:01





Mathematics, 27.08.2020 07:01

Mathematics, 27.08.2020 07:01



Mathematics, 27.08.2020 07:01

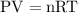 at a constant temperature,
at a constant temperature, 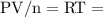 constant. That means,
constant. That means,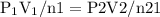
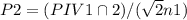
![P 2=(P 1 V 1 \cap 2) /(V 2 n 1)=\left[(155 a t m)^{*}(5.00 L)^{*}(2 m o l e s)\right] /\left[(25.00 L)^{*}(5 \text { moles }]\right]=124 \text { atm }](/tpl/images/0552/9219/932b9.png)


