
Physics, 20.03.2020 11:59 elijahbebeastin
The molar heat of fusion of benzene is kJ/mol. Its molar heat of vaporization is kJ/mol. Calculate the heat required to melt g benzene at its normal melting point. Calculate the heat required to vaporize g benzene at its normal boiling point. Why is the heat of vaporization more than three times the heat of fusion?

Answers: 3


Another question on Physics

Physics, 22.06.2019 03:00
Oxygen and nutrients are moved through the body by the: a. circulatory system c. lymphatic system b. digestive system d. reproductive system
Answers: 1

Physics, 22.06.2019 17:40
A15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°c to 175°c. what is the specific heat capacity of iron?
Answers: 1

Physics, 23.06.2019 02:30
Anthropology is the study of the effects of disease on the body, while forensic anthropology studies the disease of crime a.) true b.) false
Answers: 2

Physics, 23.06.2019 04:40
Asign is hung between two cables as illustrated below. if the sign weighs 350 n, what is the tension (in n) in each cable?
Answers: 1
You know the right answer?
The molar heat of fusion of benzene is kJ/mol. Its molar heat of vaporization is kJ/mol. Calculate...
Questions

Mathematics, 04.02.2020 14:59




Mathematics, 04.02.2020 14:59



Social Studies, 04.02.2020 14:59

Mathematics, 04.02.2020 14:59

Mathematics, 04.02.2020 14:59


Mathematics, 04.02.2020 15:00

Mathematics, 04.02.2020 15:00

Mathematics, 04.02.2020 15:00

Business, 04.02.2020 15:00

History, 04.02.2020 15:00

Business, 04.02.2020 15:00

History, 04.02.2020 15:00


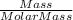 which is
which is 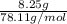 = 0.1056 moles
= 0.1056 moles

