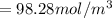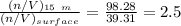Physics, 23.03.2020 16:54 Jamilia561
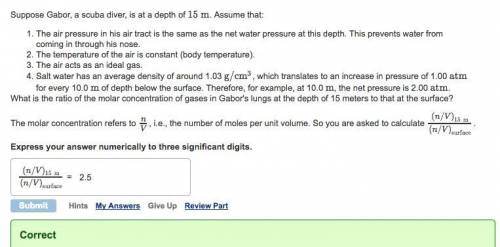
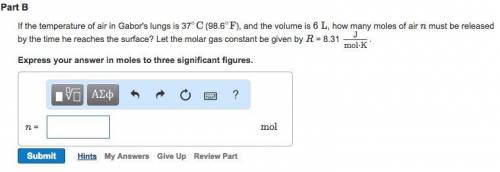
Suppose Gabor, a scuba diver, is at a depth of 15m. Assume that: The air pressure in his air tract is the same as the net water pressure at this depth. This prevents water from coming in through his nose. The temperature of the air is constant (body temperature). The air acts as an ideal gas. Salt water has an average density of around 1.03 g/cm3, which translates to an increase in pressure of 1.00 atm for every 10.0 m of depth below the surface. Therefore, for example, at 10.0 m, the net pressure is 2.00 atm. What is the ratio of the molar concentration of gases in Gabor's lungs at the depth of 15 meters to that at the surface

Answers: 1


Another question on Physics

Physics, 22.06.2019 07:10
Road users moving into your lane, brake lights, and abrupt changes in road surface are a. rare at night b. indicators of potential hazards c. not worth worrying about before you reach them d. no problem for experienced drivers
Answers: 1


Physics, 22.06.2019 10:00
(a) calculate the number of electrons in a small, electrically neutral silver pin that has a mass of 10.0 g. silver has 47 electrons per atom, and its molar mass is 107.87 g/mol. (b) imagine adding electrons to the pin until the negative charge has the very large value 1.00 mc. how many electrons are added for every 109 electrons already present
Answers: 3

Physics, 22.06.2019 13:20
Arrange the images in order to show how lake-effect snow occurs.
Answers: 2
You know the right answer?
Suppose Gabor, a scuba diver, is at a depth of 15m. Assume that: The air pressure in his air tract i...
Questions


History, 10.06.2020 20:57


Mathematics, 10.06.2020 20:57

Mathematics, 10.06.2020 21:57


Mathematics, 10.06.2020 21:57

Mathematics, 10.06.2020 21:57



Physics, 10.06.2020 21:57

Mathematics, 10.06.2020 21:57



Mathematics, 10.06.2020 21:57



Mathematics, 10.06.2020 21:57

Biology, 10.06.2020 21:57

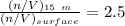
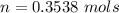
![P_d = [\frac{15m}{10m} ] (1 atm) + 1 atm](/tpl/images/0558/9055/c032e.png)
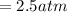
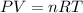
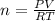
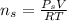
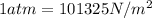 for
for 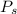 ,
,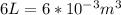 for volume , 8.314 J/mol. K for R , (37° +273) K for T into the equation
for volume , 8.314 J/mol. K for R , (37° +273) K for T into the equation 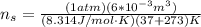
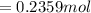
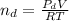
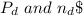 are pressure and no of moles at the depth of the water
are pressure and no of moles at the depth of the water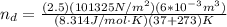
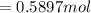
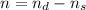
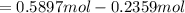
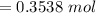
![[\frac{n}{V} ]_{surface} = \frac{0.2359mol}{6*10^-3m^3}](/tpl/images/0558/9055/08954.png)
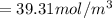
![[\frac{n}{V} ]_{15m} = \frac{0.5897}{6*10^{-3}}](/tpl/images/0558/9055/d7097.png)