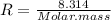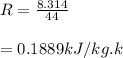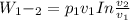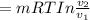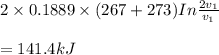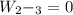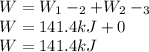Mass of 4 kilograms of carbon dioxide (CO2) in a piston-cylinder assembly undergoes two processes in series from an initial state where p1 = 0.5 MPa, T1 = 267°C: Process 1–2: Constant-temperature expansion until the volume is twice the initial volume. Process 2–3: Constant-volume heating until the pressure is again 0.5 MPa. Assuming ideal gas behavior, determine the overall work, in kJ.

Answers: 3


Another question on Physics

Physics, 21.06.2019 18:20
Let f(x) = x4- 8x2 . find the relativeextrema of this function using the second derivative test.
Answers: 2

Physics, 21.06.2019 21:00
50 g of ice at 0°c is dropped in a beaker containing 100 g of water at 0°c. what will be the contents of the beaker after 5 hours? assume that the room temperature is 0°c.
Answers: 1


Physics, 22.06.2019 18:50
8.29 two streams containing pyridine and acetic acid at 25°c are mixed and fed into a heat exchanger. due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°c using a stream of chilled ethylene glycol as indicated in the diagram. calculate the mass flow rate of ethylene glycol needed. the heat capacity of ethylene glycol at these conditions is approximately 2.8 kj/(kg k), and the enthalpy change of mixing (δmixh) is given below.
Answers: 3
You know the right answer?
Mass of 4 kilograms of carbon dioxide (CO2) in a piston-cylinder assembly undergoes two processes in...
Questions


English, 06.12.2021 08:10


Chemistry, 06.12.2021 08:10

Social Studies, 06.12.2021 08:10

History, 06.12.2021 08:10


Mathematics, 06.12.2021 08:20

Mathematics, 06.12.2021 08:20


Mathematics, 06.12.2021 08:20

Mathematics, 06.12.2021 08:20

Mathematics, 06.12.2021 08:20

Mathematics, 06.12.2021 08:20

Mathematics, 06.12.2021 08:20

Mathematics, 06.12.2021 08:20


Mathematics, 06.12.2021 08:20

Mathematics, 06.12.2021 08:20

History, 06.12.2021 08:20