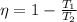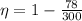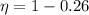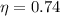Physics, 30.03.2020 23:23 greend0719
Imagine you use Nitrogen as your gas. If you have the cold side as cold as you can without liquefying it (78 K), and run the hot side at room temperature (300 K) what is the efficiency of a Stirling engine

Answers: 2


Another question on Physics

Physics, 21.06.2019 17:20
When an object moves in uniform circular motion, the direction of its acceleration is a) in the same direction as its velocity vector. b) in the opposite direction of its velocity vector c) is directed toward the center of its circular path. d) is directed away from the center of its circular path. e) depends on the speed of the object.
Answers: 1

Physics, 22.06.2019 07:50
Calculate the ratio of h+ ions to oh– ions at a ph = 6. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 6. are they the same? why or why not? record your explanation in table a. what is the concentration of h+ ions at a ph = 6? mol/l what is the concentration of oh– ions at a ph = 6? mol/l what is the ratio of h+ ions to oh– ions at a ph = 6? : 1
Answers: 1

Physics, 22.06.2019 08:00
Who was the first scientist to measure speed as distance over time?
Answers: 1

Physics, 22.06.2019 12:10
Consider a one meter long horizontal pipe with a constant 100 cm^2 cross sectional area. water flows rightward into the pipe at x = 0 with flow velocity 02m/sec at every point within the pipe intake area. at x=1, the rightward flow rate is 0.192 m/sec. assume the water is a conserved quantity in the pipe, so there must be a leak (a sink) somewhere in the pipe. 1. compute net volumetric flow of the source if the system to be in equilibrium. 2. now assume the pipe in the problem has no leaks. compute the net volumetric rate of change for the system.
Answers: 3
You know the right answer?
Imagine you use Nitrogen as your gas. If you have the cold side as cold as you can without liquefyin...
Questions

Arts, 30.07.2019 12:00






Computers and Technology, 30.07.2019 12:00

Social Studies, 30.07.2019 12:00


Computers and Technology, 30.07.2019 12:00

Mathematics, 30.07.2019 12:00

Computers and Technology, 30.07.2019 12:00

Computers and Technology, 30.07.2019 12:00

Biology, 30.07.2019 12:00

Computers and Technology, 30.07.2019 12:00

History, 30.07.2019 12:00


Biology, 30.07.2019 12:00

Mathematics, 30.07.2019 12:00

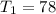 K
K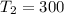 K
K