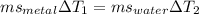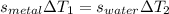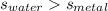Physics, 31.03.2020 20:31 tkdurocher
A 50-g chunk of a common metal at 80°C is placed into a calorimeter with 50 g of water at 50°C. At thermal equilibrium, which effect would be most likely?
Group of answer choices
The water will have changed temperature more than the metal.
The metal will have changed temperature more than the water.
The water will have gained more thermal energy than the metal lost.
The equilibrium temperature will be the average of 65°C.

Answers: 3


Another question on Physics

Physics, 21.06.2019 23:30
If 21.2 kcals of energy is released by this reaction, how many kj does the reaction release? (1 cal 4.184 j)
Answers: 1

Physics, 21.06.2019 23:50
Select the correct answer from each drop-down menu. compared to its surroundings, the concentration of solutes is low inside a cell. so, the cell is with respect to its surroundings. a particular solute in this cell uses energy for its transport from the cell to its surroundings. this type of transport is called
Answers: 3

Physics, 22.06.2019 04:30
The image shows the positions of a car on a roller coaster track. arrange the cars in order based on their gravitational potential energy. begin with the lowest potential energy and end with the highest.
Answers: 1

Physics, 22.06.2019 05:00
He viscosities of several liquids are being compared. all the liquids are poured down a slope with equal path lengths. the liquid with the highest viscosity will
Answers: 3
You know the right answer?
A 50-g chunk of a common metal at 80°C is placed into a calorimeter with 50 g of water at 50°C. At t...
Questions

Mathematics, 29.01.2020 15:59


Mathematics, 29.01.2020 15:59



English, 29.01.2020 15:59

Mathematics, 29.01.2020 16:00

English, 29.01.2020 16:00

Biology, 29.01.2020 16:00


Mathematics, 29.01.2020 16:00


Biology, 29.01.2020 16:00



Mathematics, 29.01.2020 16:00

English, 29.01.2020 16:00

Mathematics, 29.01.2020 16:00


English, 29.01.2020 16:00