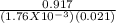Physics, 31.03.2020 23:35 kayonapretty14p45995
Gaseous carbon dioxide is partially decomposed according to the following equation. An initial pressure of 1.00 atm of CO2 is placed in a closed container at 2500 K, and 2.1 % of the molecules decompose. Determine the equilibrium constant Kp at this temperature

Answers: 1


Another question on Physics

Physics, 22.06.2019 05:10
Which situation will have the highest resistance? a.long wire and high temperatureb.short wire and high temperaturec.long wire and cold temperaturedshort wire and low temperature
Answers: 2

Physics, 22.06.2019 07:50
The ratio of lift to drag l/d for a wing or airfoil is an important aerodynamic parameter, indeed, it is a direct measure of the aerodynamic efficiency of the wing. if a wing is pitched through a range of angle of attack, l/d first increases, then goes through a maximum, and then decreases. consider an infinite wing with an naca 2412 airfoil. estimate the maximum value of l/d. assume that the reynolds number is 9x10^6.
Answers: 2

Physics, 22.06.2019 08:50
The electronic structure or chlorine is 2.8.7 what is the electronic structure of fluorine?
Answers: 2

Physics, 22.06.2019 13:30
6–43 a food department is kept at 2128c by a refrigerator in an environment at 308c. the total heat gain to the food department is estimated to be 3300 kj/h and the heat rejection in the condenser is 4800 kj/h. determine the power input to the compressor, in kw and the cop of the refrigerator.
Answers: 2
You know the right answer?
Gaseous carbon dioxide is partially decomposed according to the following equation. An initial press...
Questions


Mathematics, 10.07.2019 05:00



Mathematics, 10.07.2019 05:00

Mathematics, 10.07.2019 05:00


Mathematics, 10.07.2019 05:00


English, 10.07.2019 05:00




Mathematics, 10.07.2019 05:00

English, 10.07.2019 05:00





Mathematics, 10.07.2019 05:00

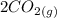 ⇄
⇄ 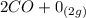
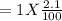

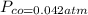
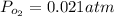
![Kp = \frac{[CO_{2} ]^{2} }{[CO]^{2} [O_{2} ] }](/tpl/images/0574/3678/cce9a.png)
![Kp = \frac{[0.958 ]^{2} }{[0.042]^{2} [0.021] }](/tpl/images/0574/3678/1e9e8.png)