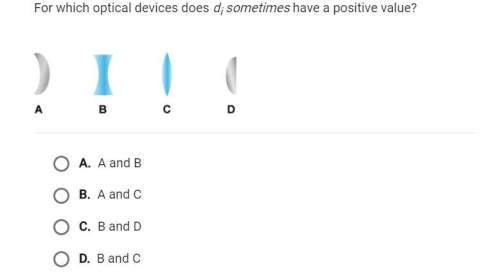
During inhalation, a person's diaphragm and intercostal muscles contract, expanding the chest cavity and lowering the internal air pressure
below ambient so that air flows in through the mouth and nose to the lungs. Suppose a person's lungs hold 1300 mL of air at a pressure of
1.00 atm. If they expand their chest cavity by 460 mL while keeping their nose and mouth closed so that no air is inhaled, what will be the air
pressure in their lungs in atm? Assume the air temperature remains constant.

Answers: 1


Another question on Physics

Physics, 22.06.2019 12:20
Which lists the pairs of plates in order from least to greatest in terms of the work done to move the electron?
Answers: 2


Physics, 22.06.2019 17:00
Since the energy was not raising the temperature of the water during these segments, what was the energy used to do instead? your answer must include an explanation of what is happening to the molecules.
Answers: 3

Physics, 22.06.2019 17:30
Ethanol has a heat of vaporization of 38.56 kj/mol and a normal boiling point of 78.4 c. what is the vapor pressure of ethanol at 14 c?
Answers: 3
You know the right answer?
During inhalation, a person's diaphragm and intercostal muscles contract, expanding the chest cavity...
Questions

Mathematics, 01.01.2022 23:40


Mathematics, 01.01.2022 23:40



Mathematics, 01.01.2022 23:40

Mathematics, 01.01.2022 23:40




English, 01.01.2022 23:40

Mathematics, 01.01.2022 23:40


English, 01.01.2022 23:50


English, 01.01.2022 23:50

Mathematics, 01.01.2022 23:50

History, 01.01.2022 23:50

Mathematics, 01.01.2022 23:50