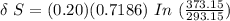Physics, 07.04.2020 15:51 marioruiz7944
0.2 kg of air is heated in a constant volume process from 20 to 100(degrees celsius). The specific heat at constant volume is 0.7186J/ Kg K. Calculate the change in entropy of this process.

Answers: 2


Another question on Physics


Physics, 22.06.2019 23:00
Imagine an isolated positive point charge q (many times larger than the charge on a single proton). there is a charged particle a (whose charge is much smaller than charge q) at a distance from the point charge q. on which of the following quantities does the magnitude of the electric field created by charge q at particle a's position depend? check all that apply.the type of the charge on the charged particle athe relative orientation between q and a (while the distance between q and a is fixed)the specific location of the charged particle a (while the distance between q and a is fixed)the amount of the charge on the point charge qthe specific location of the point charge q (while the distance between q and a is fixed)the distance between the point charge q and the charged particle athe amount of the charge on the charged particle a
Answers: 3

Physics, 22.06.2019 23:30
Which system of units is used by only a small number of countries in the world, including the u.s.
Answers: 3

Physics, 23.06.2019 01:00
Two solutions of the same uv-absorbing molecule were analyzed by uv-vis spectroscopy on the same instrument using 1 cm pathlength cells. use data from the table to calculate by what percentage the concentration of solution b is compared to that of solution a.sample absorbance at 315nm a 0.45 b 0.80
Answers: 1
You know the right answer?
0.2 kg of air is heated in a constant volume process from 20 to 100(degrees celsius). The specific h...
Questions

Mathematics, 12.01.2021 23:40

Mathematics, 12.01.2021 23:40

Mathematics, 12.01.2021 23:40

Mathematics, 12.01.2021 23:40

Mathematics, 12.01.2021 23:40



Arts, 12.01.2021 23:40

Mathematics, 12.01.2021 23:40

Mathematics, 12.01.2021 23:40

Mathematics, 12.01.2021 23:40

Mathematics, 12.01.2021 23:40

Mathematics, 12.01.2021 23:40

Mathematics, 12.01.2021 23:40


English, 12.01.2021 23:40

Arts, 12.01.2021 23:40



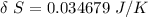
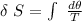
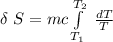
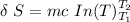
![\delta \ S = mc \ [In \ (T_2) - In \ ({T_1})]](/tpl/images/0586/3826/760c7.png)
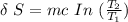
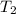 = 100° C = ( 100 + 273.15) = 373.15 K
= 100° C = ( 100 + 273.15) = 373.15 K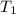 = 20° C = ( 20 + 273.15) = 293.15 K
= 20° C = ( 20 + 273.15) = 293.15 K