
Physics, 07.04.2020 16:01 rileyeddins1010
A fixed amount of a particular ideal gas at 168C and a pressure of 1.75 3 105 Pa occupies a volume of 2.75 m3. If the volume is increased to 4.20 m3 and the temperature is raised to 26.48C, what will be the new pressure of the gas?

Answers: 3


Another question on Physics

Physics, 21.06.2019 21:50
When applying kirchhoff's rules, one of the essential steps is to mark each resistor with plus and minus signs to label how the electric potential changes from one end of the resistor to the other. the circuit in the drawing contains four resistors, each marked with the associated plus and minus signs. however, one resistor is marked incorrectly. which one is it?
Answers: 1

Physics, 21.06.2019 22:30
Laboratory experiments, observational field studies, and model-building are all examples of different forms of scientific investigations. in what way do laboratory experiments primarily differ from other forms of scientific investigations? a. a laboratory experiment is the only accepted form of investigation within the scientific community. b. studies about how things behave in nature or studies involving very large objects are best answered through laboratory experiments. c. laboratory experiments involve the identification and control of variables. d. data can only be generated through laboratory experiments, not other forms of investigation.
Answers: 3

Physics, 21.06.2019 23:30
Jessie ran 5000 meters from the cops and an average speed of 6 meters / second before he got caught . how long did he run?
Answers: 1

Physics, 22.06.2019 00:00
Name three different units of energy used to measure heat and describe what type of situations each is usually used.
Answers: 2
You know the right answer?
A fixed amount of a particular ideal gas at 168C and a pressure of 1.75 3 105 Pa occupies a volume o...
Questions

Business, 16.09.2019 12:00

Biology, 16.09.2019 12:00


Physics, 16.09.2019 12:00


Social Studies, 16.09.2019 12:00


History, 16.09.2019 12:00


History, 16.09.2019 12:00


Computers and Technology, 16.09.2019 12:00

Mathematics, 16.09.2019 12:00

Health, 16.09.2019 12:00

Mathematics, 16.09.2019 12:00


Mathematics, 16.09.2019 12:00



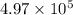 Pa
Pa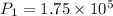 Pa
Pa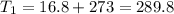 K
K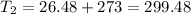 K
K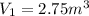
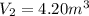
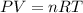
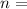 number of moles, here
number of moles, here 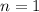 ,
, 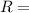 gas constant
gas constant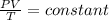
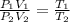
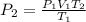
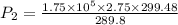
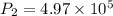 Pa
Pa

