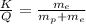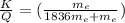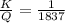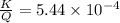Physics, 07.04.2020 23:03 blaizelange2573
7. A neutron at rest decays (breaks apart) into a proton and an electron. Energy is released in the decay, in the form of kinetic energy of the proton and electron. The mass of a proton is 1836 times the mass of an electron. What fraction of the total energy released goes into the kinetic energy of the proton?

Answers: 1


Another question on Physics

Physics, 21.06.2019 17:10
Aspecial electronic sensor is embedded in the seat of a car that takes riders around a circular loop-the-loop ride at an amusement park. the sensor measures the magnitude of the normal force that the seat exerts on a rider. the loop-the-loop ride is in the vertical plane and its radius is 23 m. sitting on the seat before the ride starts, a rider is level and stationary, and the electronic sensor reads 740 n. at the top of the loop, the rider is upside down and moving, and the sensor reads 370 n. what is the speed of the rider at the top of the loop?
Answers: 1

Physics, 22.06.2019 09:30
Which are advantages of renewable resources? check all that apply. renewable energy supplies are completely reliable everywhere. some renewable resources will never be used up. little or no waste is produced by renewable resource plants. electricity can be generated in large quantities. many renewable energy facilities have lower operating costs.
Answers: 1

Physics, 22.06.2019 11:20
More solar radiation is absorbed by earth’s surface than by
Answers: 1

Physics, 22.06.2019 13:40
What is the thinnest soap film (excluding the case of zero thickness) that appears black when illuminated with light with a wavelength of 480 ? the index of refraction of the film is 1.34, and there is air on both sides of the film. express your answer in nanometers. hint 1. how to approach th
Answers: 1
You know the right answer?
7. A neutron at rest decays (breaks apart) into a proton and an electron. Energy is released in the...
Questions


Mathematics, 19.07.2019 17:30




Biology, 19.07.2019 17:30

Chemistry, 19.07.2019 17:30

Business, 19.07.2019 17:30


Computers and Technology, 19.07.2019 17:30




History, 19.07.2019 17:30

Arts, 19.07.2019 17:30

Mathematics, 19.07.2019 17:30

Spanish, 19.07.2019 17:30


Biology, 19.07.2019 17:30

Geography, 19.07.2019 17:30

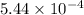
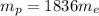
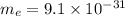 kg
kg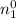 ⇄
⇄ 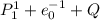
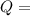 energy released
energy released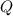 value of this reaction is given by
value of this reaction is given by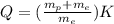
 kinetic energy of reaction
kinetic energy of reaction