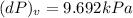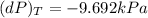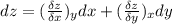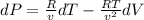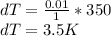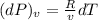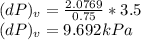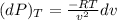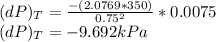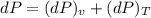Physics, 15.04.2020 00:41 muhammadcorley123456
Consider helium at 350 K and 0.75 m3/kg. Using Eq. 12-3, determine the change in pressure corresponding to an increase of (a) 1 percent in temperature at constant specific volume, (b) 1 percent in specific volume at constant temperature, and (c) 1 percent in both the temperature and specific volume.

Answers: 3


Another question on Physics

Physics, 22.06.2019 00:30
Order the sequence of ideas that lead to marie curies discovery of radioactive elements number the events in chronological order starting with the oldest
Answers: 2

Physics, 22.06.2019 03:00
1. a net force of 100 newton’s is applied to a wagon for 5 seconds. this causes the wagon to undergo a change in momentum of
Answers: 2

Physics, 22.06.2019 05:30
The volume of a gas is halved during an adiabatic compression that increases the pressure by a factor of 2.5. what is the specific heat ratio? show the math steps .
Answers: 3

Physics, 22.06.2019 12:50
Assume you measured the mass of the cart to be (500 ± 1) g and the mass of the additional mass you put on the cart to be (500 ± 1) g as well. since the scale you are using in the lab cannot measure objects heavier than 600g you will have to sum up individual pieces and propagate the error. so what would be the mass and the standard error of the cart and the mass
Answers: 3
You know the right answer?
Consider helium at 350 K and 0.75 m3/kg. Using Eq. 12-3, determine the change in pressure correspond...
Questions


History, 22.04.2021 01:00


Physics, 22.04.2021 01:00

Mathematics, 22.04.2021 01:00

Mathematics, 22.04.2021 01:00



Mathematics, 22.04.2021 01:00

Chemistry, 22.04.2021 01:00




Social Studies, 22.04.2021 01:00


Social Studies, 22.04.2021 01:00


Mathematics, 22.04.2021 01:10

Mathematics, 22.04.2021 01:10

Mathematics, 22.04.2021 01:10