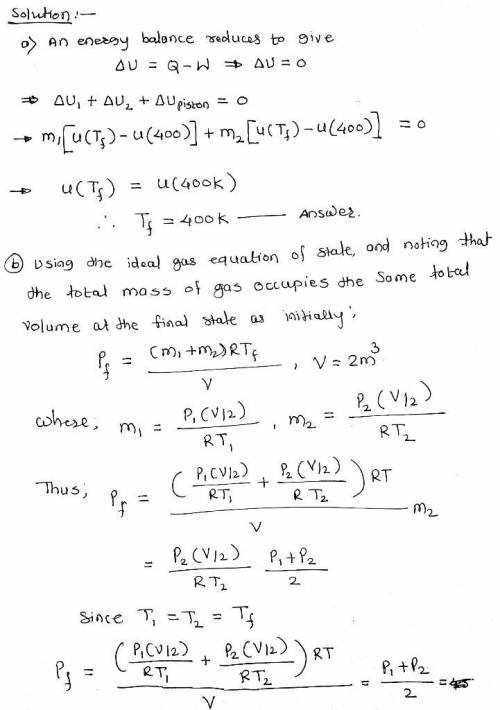
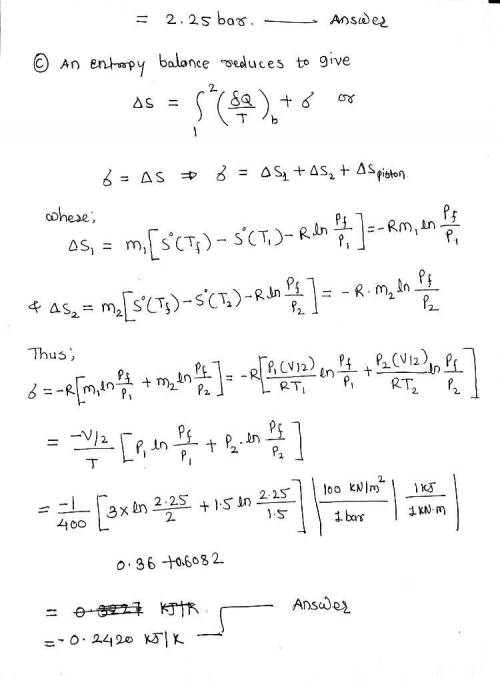
Of the piston is 1.0 m3 of air at 400 K, 3 bar. On the other side is 1.0 m3 of air at 400 K, 1.5 bar. The piston is released and equilibrium is attained, with the piston experiencing no change of state. Employing the ideal gas model for the air, determine a. the final temperature of the air, in K. b. the final pressure of the air, in bar. c. the amount of entropy produced, in kJ/K

Answers: 2


Another question on Physics

Physics, 22.06.2019 04:30
You are traveling in a car going at a constant speed of 100km/h down a long, straight highway. you pass another car going in the same direction which is traveling at a constant speed of 80km/h. as measured from your car's reference frame this other car is traveling at −20km/h. what is the acceleration of your car as measured from the other car's reference frame? what is the acceleration of the other car as measured from your car's reference frame?
Answers: 2

Physics, 22.06.2019 08:00
5g of ammonium nitrate was dissolved in 60g of water in an insulated container. the temperature at the start of the reaction was 23.0°c and at the end it was 19.0°c. calculate the energy absorbed by the reaction.
Answers: 3

Physics, 22.06.2019 08:30
An object weigh 40n in air ,weigh 20n when submerged in water,and 30n when submerged in a liquid of unknown liquid density.what is the density of unknown of liquid?
Answers: 1

Physics, 22.06.2019 12:20
Which of the following situations is impossible? a) an object has velocity directed east and acceleration directed east. b) an object has zero velocity but non-zero acceleration. c) an object has constant non-zero velocity and changing acceleration. d) an object has velocity directed east and acceleration directed west. e) an object has constant non-zero acceleration and changing velocity.
Answers: 2
You know the right answer?
Of the piston is 1.0 m3 of air at 400 K, 3 bar. On the other side is 1.0 m3 of air at 400 K, 1.5 bar...
Questions

Business, 24.07.2019 16:30



Mathematics, 24.07.2019 16:30

Social Studies, 24.07.2019 16:30



History, 24.07.2019 16:30

Arts, 24.07.2019 16:30

History, 24.07.2019 16:30


Biology, 24.07.2019 16:30

Mathematics, 24.07.2019 16:30

History, 24.07.2019 16:30




History, 24.07.2019 16:30

History, 24.07.2019 16:30