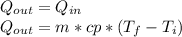reaches an equilibrium temperature of 31.1°C.

Physics, 17.04.2020 06:47 johnisawesome999
A jar of tea is placed in sunlight until it
reaches an equilibrium temperature of 31.1°C.
In an attempt to cool the liquid, which has a
mass of 177 g. 110 g of ice at 0.0°C is added.
At the time at which the temperature of the
tea is 29.1°C, find the mass of the remaining ice in the jar. The specific heat of water is 4186 J/kg.°C. Assume the specific heat capacity of the tea to be that of pure liquid water. Answer in units of g.

Answers: 2


Another question on Physics


Physics, 21.06.2019 18:00
Assuming weightless pulleys and 100% efficiency, what is the minimum input force required to lift a 120 n weight using a single fixed pulley?
Answers: 2

Physics, 22.06.2019 11:00
Alarge box of mass m is pulled across a horizontal frictionless surface by a horizontal rope with tension t. a small box of mass m sits on top of the large box. the coefficients of static and
Answers: 1

You know the right answer?
A jar of tea is placed in sunlight until it
reaches an equilibrium temperature of 31.1°C.
reaches an equilibrium temperature of 31.1°C.
Questions


Biology, 29.12.2019 06:31


Computers and Technology, 29.12.2019 06:31

Mathematics, 29.12.2019 06:31




Mathematics, 29.12.2019 06:31

English, 29.12.2019 06:31

Biology, 29.12.2019 06:31


Mathematics, 29.12.2019 06:31

Mathematics, 29.12.2019 06:31


Mathematics, 29.12.2019 06:31

World Languages, 29.12.2019 06:31

Computers and Technology, 29.12.2019 06:31

Biology, 29.12.2019 06:31

Mathematics, 29.12.2019 06:31