
1. in a single atom, no more than 2 electrons can occupy a single orbital? a. true b. false
2. the maximum number of electrons allowed in a p sublevel of the 3rd principal level is?
a. 1 b.6 c. 2 d. 8 e. 3
3. a neutral atom has a ground state electronic configuration of 1s^2 2s^2. which of the following statements concerning this atom is/are correct? a. the atom has a total of two orbitals occupied. b. all of the above. c. the atom has no unpaired electrons. d. the atom contains 4 protons. e. the element has atomic number 4.

Answers: 3


Another question on Physics

Physics, 21.06.2019 22:30
A1200 kg car traveling north at 10 m/s is rear-ended by a 2000 kg truck traveling at 30 m/s. what is the total momentum before and after the collision?
Answers: 3

Physics, 22.06.2019 05:30
Because light travels in a straight line and casts a shadow, isaac newton hypothesized that light is
Answers: 1

Physics, 22.06.2019 15:00
When is a current produced? when the terminals of an electrochemical cell are connected by a wire if the electric circuit is opened in an electrochemical cell if the electrolyte is removed from an electrochemical cell when the electrodes are reversed in an electrochemical cell
Answers: 1

Physics, 22.06.2019 18:30
In the united states, tornadoes generally occur because of the freezing of ocean water underwater earthquakes meeting of cool and warm air masses shifting of warm and cool ocean currents
Answers: 1
You know the right answer?
1. in a single atom, no more than 2 electrons can occupy a single orbital? a. true b. false
...
...
Questions






Chemistry, 05.10.2021 14:10

English, 05.10.2021 14:10




Mathematics, 05.10.2021 14:10


Mathematics, 05.10.2021 14:10




World Languages, 05.10.2021 14:10



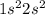 .
.

