Please help me answer this :
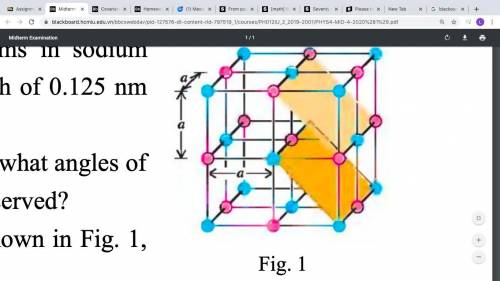
Knowing that the spacing of adjacent atoms in sodium
chlori...

Physics, 18.04.2020 07:01 tdahna0403
Please help me answer this :
Knowing that the spacing of adjacent atoms in sodium
chloride crystal is a = 0.282 nm (Fig. 1). X rays with a wavelength of 0.125 nm
are sent through this crystal.
a/ If diffraction from planes parallel to a cube face is considered, at what angles of
the incoming beam relative to the crystal planes will maxima be observed?
b/ Repeat part a/ for diffraction produced by the inclined planes shown in Fig. 1,
which are separated by a/(root of 2)

Answers: 1


Another question on Physics

Physics, 21.06.2019 18:00
1. in terms of fuel economy rank the following hybrid and electric vehicles, and justify application specific to each hybrid technology stop/start | mild hybrid | full hybrid | plug-in hybrid | full electric a. rank based on fuel economy b. justify your application specific for each technology
Answers: 3

Physics, 22.06.2019 14:00
If element x has 99 protons how many electrons does it have
Answers: 1

Physics, 22.06.2019 21:00
What do the atoms of elements in the same group have in common? a. they have the same atomic numbers. b. they have the same average atomic masses. c. they have the same number of electron shells. d. they have the same number of electrons in their outermost shells.
Answers: 1

Physics, 22.06.2019 21:30
Global climatic patterns are changing because of the melting of polar ice caps. the melting of polar ice caps is an example of the interaction between the atmosphere and cryosphere cryosphere and geosphere hydrosphere and geosphere biosphere and cryosphere
Answers: 1
You know the right answer?
Questions


Mathematics, 19.07.2020 01:01



Biology, 19.07.2020 01:01



Social Studies, 19.07.2020 01:01

Mathematics, 19.07.2020 01:01




Mathematics, 19.07.2020 01:01


Mathematics, 19.07.2020 01:01

Mathematics, 19.07.2020 01:01


Mathematics, 19.07.2020 01:01

Mathematics, 19.07.2020 01:01



