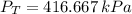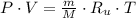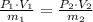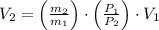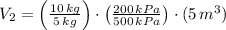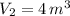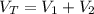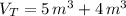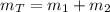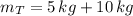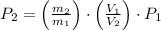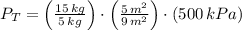Physics, 21.04.2020 16:29 herchellann302
A 5 m3 tank containing 5kg of an unknown ideal gas at 500 kPa is connected through a valve to another tank containing 10 kg of the same gas at 200 kPa. The two tanks have thermal equilibrium with the ambient environment. After the valve has been opened, the tanks again reach thermal equilibrium with their ambient environment. What is the a) total volume, b) total mass, and c) pressure of the gas in the two tanks

Answers: 1


Another question on Physics

Physics, 22.06.2019 16:50
Which best describes the first law of thermodynamics as compared to the second law of thermodynamics? a. the first law describes how thermal energy is conserved but not the direction it moves. b. the first law describes the direction thermal energy moves but not how it is conserved. c. the first law describes how thermal energy can be created but not how it can be destroyed. d. the first law describes how thermal energy can be destroyed but not how it can be created.
Answers: 1

Physics, 23.06.2019 02:00
How do you count the total number of atoms present on one side of a chemical equation?
Answers: 1


Physics, 23.06.2019 20:30
Ameteorite strikes earth and forms a crater, decreasing the meteorite's momentum to zero. does this phenomenon contradict the conservation of momentum? choose as many answers as you think are correct.
Answers: 1
You know the right answer?
A 5 m3 tank containing 5kg of an unknown ideal gas at 500 kPa is connected through a valve to anothe...
Questions



Mathematics, 24.03.2021 03:40

Mathematics, 24.03.2021 03:40

Mathematics, 24.03.2021 03:40

Mathematics, 24.03.2021 03:40




Mathematics, 24.03.2021 03:40

English, 24.03.2021 03:40

Mathematics, 24.03.2021 03:40

Mathematics, 24.03.2021 03:40

Mathematics, 24.03.2021 03:40



History, 24.03.2021 03:40


Medicine, 24.03.2021 03:40

Mathematics, 24.03.2021 03:40

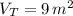 , b)
, b) 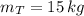 , c)
, c)