
Physics, 21.04.2020 17:34 toddbecca9
An early model of the atom, proposed by Rutherford after his discovery of the atomicnucleus, had a positive point charge +Ze(the nucleus) at the center of a sphere ofradiusRwith uniformly distributed negative charge−Ze. Zis the atomic number, thenumber of protons in the nucleus and the number of electrons in the negative sphere.
a. Show that the electric field inside this atom is
b. What is the electric field at the surface of the atom? Is this the expected value? Explain.
c. A uranium atom has Z = 92 and R = 0.10 nm. What is the electric field at r = R/2?

Answers: 2


Another question on Physics

Physics, 21.06.2019 20:20
Suppose that a comet that was seen in 563 a.d. by chinese astronomers was spotted again in year 1951. assume the time between observations is the period of the comet and take its eccentricity as 0.986. what are (a) the semimajor axis of the comet's orbit and (b) its greatest distance from the sun?
Answers: 1

Physics, 22.06.2019 07:30
Choose all the answers that apply. our solar system: is in a spiral galaxy no longer includes pluto is made mostly of empty space is in the andromeda galaxy is the only known system that supports life
Answers: 3

Physics, 22.06.2019 11:10
An isotope undergoes radioactive decay by emitting radiation that has a –1 charge. what other characteristic does the radiation have?
Answers: 3

Physics, 22.06.2019 13:00
Nacidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused the anode to lose 0.584 g after 1.52 ✕ 103 s. given that the charge of an electron is 1.6022 ✕ 10−19 c, calculate avogadro's number. assume that copper is oxidized to cu2+ ions.
Answers: 1
You know the right answer?
An early model of the atom, proposed by Rutherford after his discovery of the atomicnucleus, had a p...
Questions


Biology, 10.04.2020 05:14

Mathematics, 10.04.2020 05:14

Mathematics, 10.04.2020 05:14



Mathematics, 10.04.2020 05:14

Mathematics, 10.04.2020 05:14

Mathematics, 10.04.2020 05:14

Mathematics, 10.04.2020 05:14





Spanish, 10.04.2020 05:14




Mathematics, 10.04.2020 05:14

Mathematics, 10.04.2020 05:14

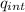 / ε₀
/ ε₀

