
Physics, 21.04.2020 19:11 leoniandreww
In an adiabatic process oxygen gas in a container is compressed along a path that can be described by the following pressure p, in atm, as a function of volume V, in liters: p = p0 V-6/5. Here p0 is a constant of units atm⋅L6/5. show answer Incorrect Answer 50% Part (a) Write an expression for the work W done on the gas when the gas is compressed from a volume Vi to a volume Vf.

Answers: 1


Another question on Physics

Physics, 21.06.2019 21:10
State one advantage and one disadvantage of using a plane mirrior as a driving mirrior
Answers: 1

Physics, 22.06.2019 02:00
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd question 6 options: 3000 joules 30 joules 320 joules 3200 joules
Answers: 2


Physics, 22.06.2019 19:10
Global warming will produce rising sea levels partly due to melting ice caps but also due to the expansion of water as average ocean temperatures rise. to get some idea of the size of this effect, calculate the change in length (in m) of a column of water 1.45 km high for a temperature increase of 1.12°c. assume the column is not free to expand sideways. as a model of the ocean, that is a reasonable approximation, as only parts of the ocean very close to
Answers: 3
You know the right answer?
In an adiabatic process oxygen gas in a container is compressed along a path that can be described b...
Questions



Business, 24.07.2019 17:40





Mathematics, 24.07.2019 17:40






Spanish, 24.07.2019 17:40

Physics, 24.07.2019 17:40

Biology, 24.07.2019 17:40




Biology, 24.07.2019 17:40

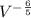 dV
dV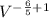 / (
/ ( 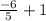 )
)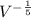
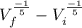 ) ltr- atm.
) ltr- atm. 

