
Physics, 06.05.2020 03:01 daisyramirez2057
A 6.00 kg piece of solid copper metal at an initial temperature T is placed with 2.00 kg of ice that is initially at -10.0 ∘C. The ice is in an insulated container of negligible mass and no heat is exchanged with the surroundings. After thermal equilibrium is reached, there is 0.50 kg of ice and 1.50 kg of liquid water.
Required:
What was the initial temperature of the piece of copper?

Answers: 1


Another question on Physics

Physics, 22.06.2019 09:10
Which lists the organs in the correct order as food passes from the mouth to anus?
Answers: 1

Physics, 22.06.2019 10:30
The uniform slender bar rests on a smooth horizon- tal surface when a force f is applied normal to the bar at point a. point a is observed to have an initial acceleration aa of 20 m/s2, and the bar has a corre- sponding angular acceleration of 18 rad /s2. deter- mine the distance b.
Answers: 1


Physics, 22.06.2019 17:00
If a negatively charged particle is placed at rest in an electric potential field that increases in the positive x-direction, what will the particle do? a. accelerate in the positive x-direction b. remain at rest c. accelerate in the negative x-direction
Answers: 3
You know the right answer?
A 6.00 kg piece of solid copper metal at an initial temperature T is placed with 2.00 kg of ice that...
Questions



World Languages, 31.08.2019 21:30

Geography, 31.08.2019 21:30

History, 31.08.2019 21:30


Mathematics, 31.08.2019 21:30



Social Studies, 31.08.2019 21:30

Geography, 31.08.2019 21:30


English, 31.08.2019 21:30

History, 31.08.2019 21:30

Business, 31.08.2019 21:30

History, 31.08.2019 21:30

Mathematics, 31.08.2019 21:30



English, 31.08.2019 21:30

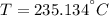
![(6000\,g) \cdot \left(0.385\,\frac{J}{g\cdot ^{\textdegree}C} \right)\cdot (T -0^{\textdegree}C ) = (2000\,g)\cdot \left(2.108\,\frac{J}{g\cdot ^{\textdegree}C} \right)\cdot [0^{\textdegree}C -(-10^{\textdegree}C)] + (1500\,g)\cdot \left(334\,\frac{J}{g} \right)](/tpl/images/0645/5025/aa197.png)


