
Physics, 06.05.2020 00:25 montamonta0204
The value of specific heat for copper is 390 J/kg⋅C∘, for aluminun is 900 J/kg⋅C∘, and for water is 4186 J/kg⋅C∘.
What will be the equilibrium temperature when a 235 g block of copper at 255 ∘C is placed in a 135 g aluminum calorimeter cup containing 825 g of water at 16.0 ∘C? Express your answer using three significant figures.

Answers: 3


Another question on Physics

Physics, 22.06.2019 06:20
Oxygen undergoes an isentropic expansion from 120 degree c and 280 kpa absolute to 140 kpa absolute. determine the final temperature of the oxygen and the amount of work per kg of o_2 produced in the process if it is adiabatic.
Answers: 1

Physics, 22.06.2019 12:30
Which levels of government establish and implement educational requirements for minors
Answers: 1

Physics, 22.06.2019 13:20
Ahanging spring stretches by 35.0 cm when an object of mass 450 g is hung on it at rest. in this situation, we define its position as x = 0. the object is pulled down an additional 18.0 cm and released from rest to oscillate without friction. what is its position x at a moment 84.4 s later? express your answer in cm.
Answers: 1

Physics, 22.06.2019 16:40
The force needed to overcome static friction is usually less than that needed to overcome kinetic friction.true or false?
Answers: 1
You know the right answer?
The value of specific heat for copper is 390 J/kg⋅C∘, for aluminun is 900 J/kg⋅C∘, and for water is...
Questions




Chemistry, 02.10.2019 10:30




Chemistry, 02.10.2019 10:30

Mathematics, 02.10.2019 10:30



History, 02.10.2019 10:30


History, 02.10.2019 10:30

Mathematics, 02.10.2019 10:30



Biology, 02.10.2019 10:30

History, 02.10.2019 10:30

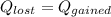
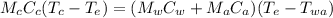
![0.235 \times 390\times(255-T_e)=([0.825\times4186]+[0.135\times900])(T_e -16)\\\\91.65(255-T_e)=(3453.45+121.5)(T_e -16)\\\\23370.75-91.65T_e=3574.95(T_e -16)\\\\23370.75-91.65T_e=3574.95T_e -57199.2\\\\3574.95T_e+91.65T_e=23370.75+57199.2\\\\3666.6T_e=80569.95\\\\T_e=\frac{80569.95}{3666.6} \\\\T_e=21.97](/tpl/images/0644/2367/dd73d.png)


