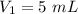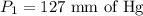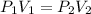A gas is placed in a 5.0-mL syringe. It exerts 127 mm Hg of pressure on the inside walls of the syringe. The syringe's plunger is pressed, reducing the volume of the syringe to 2.4 mL. The cap was not removed from the syringe, so none of the gas escapes. Assuming the temperature of the gas does not change, use Boyle's law to determine the pressure of the compressed gas.

Answers: 2


Another question on Physics

Physics, 22.06.2019 04:10
An initially unpolarized light if intensity 500 w/m^2 passes through four polarizers. the transmission axis between adjacent polarizers is 45∘. what percentage of the initial intensity is transmitted by the system?
Answers: 2

Physics, 22.06.2019 06:30
In positive numbers less than 1, the zeros between the decimal point and a non-zero number are blank significant
Answers: 1

Physics, 22.06.2019 07:00
Suppose while hauling rocks, you accidentally drop one. it breaks apart in flat, planar sections. what type of rock did you just drop? 20 points
Answers: 3

Physics, 22.06.2019 09:10
The air that we breath is made mostly of which gaseous molecule
Answers: 1
You know the right answer?
A gas is placed in a 5.0-mL syringe. It exerts 127 mm Hg of pressure on the inside walls of the syri...
Questions



Mathematics, 05.05.2020 09:09

Mathematics, 05.05.2020 09:09






Physics, 05.05.2020 09:09

Spanish, 05.05.2020 09:09



Mathematics, 05.05.2020 09:09



Chemistry, 05.05.2020 09:09

Mathematics, 05.05.2020 09:09