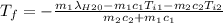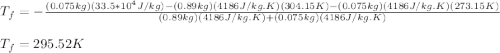Physics, 06.05.2020 04:44 Artemis3821
A thermos contains m1 = 0.89 kg of tea at T1 = 31° C. Ice (m2 = 0.075 kg, T2 = 0° C) is added to it. The heat capacity of both water and tea is c = 4186 J/(kg⋅K), and the latent heat of fusion for water is Lf = 33.5 × 104 J/kg. show answer No Attempt 50% Part (a) Input an expression for the final temperature after the ice has melted and the system has reached thermal equilibrium. Part (b) What is the final temperature in Kelvin?

Answers: 2


Another question on Physics


Physics, 22.06.2019 01:40
Lin yao looks at the back of a spoon. how should she describe her reflection? upside down and smaller upside down and larger right-side up and smaller right-side up and larger
Answers: 1

Physics, 22.06.2019 08:00
What is the average speed of a car that travels 40mph for 1 hour and 60 mph in another hour will mark brainliest
Answers: 1

Physics, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
A thermos contains m1 = 0.89 kg of tea at T1 = 31° C. Ice (m2 = 0.075 kg, T2 = 0° C) is added to it....
Questions





Mathematics, 30.03.2021 03:40


Mathematics, 30.03.2021 03:40


Mathematics, 30.03.2021 03:40

Mathematics, 30.03.2021 03:40

Mathematics, 30.03.2021 03:40


History, 30.03.2021 03:40

Mathematics, 30.03.2021 03:40


Biology, 30.03.2021 03:40



Chemistry, 30.03.2021 03:40

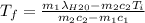
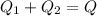
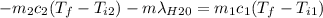 ( 1 )
( 1 )