
Physics, 07.05.2020 12:02 Lenaaa2019
Once you’ve identified the initial and final conditions, you’re ready to solve for the unknown quantity in your problem. Boyle’s Law expresses the pressure-volume relationship as P1V1=P2V2, so you will need to solve this equation for the unknown quantity and then plug in your known values to calculate the unknown. What pressure would it take to compress 250. L of helium gas initially at 1.00 atm into a 2.00 L tank at constant temperature? Express your answer with the appropriate units.

Answers: 1


Another question on Physics

Physics, 21.06.2019 20:00
Spend time observing or thinking about events that involve matter and energy. which events can you explain? which events can’t you explain?
Answers: 1

Physics, 22.06.2019 11:50
The scalar triple product computes the magnitude m of the moment of a force vector f about a specified line. it is m = (r × f) · n, where r is the position vector from the line to the point of application of the force and n is a unit vector in the direction of the line. use matlab to compute the magnitude m for the case where f = [12, −5, 4] n, r = [−3, 5, 2] m, and n = [6, 5, −7].
Answers: 3

Physics, 22.06.2019 15:30
How many neutrons does element x if it’s atomic number is 28 and its mass number is 87
Answers: 1

Physics, 22.06.2019 22:00
Four friends, a, b, c, and d are standing varying distances away from a speaker which is producing sound waves.
Answers: 2
You know the right answer?
Once you’ve identified the initial and final conditions, you’re ready to solve for the unknown quant...
Questions

History, 28.01.2020 19:04




Chemistry, 28.01.2020 19:04



English, 28.01.2020 19:04




History, 28.01.2020 19:04

Chemistry, 28.01.2020 19:04

Mathematics, 28.01.2020 19:04



Mathematics, 28.01.2020 19:05



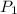 = 1 atm
= 1 atm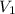 = 250L
= 250L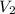 =2L
=2L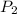 =?
=?

