
Physics, 21.05.2020 22:02 sneakypanther19
The entropy of steam increases in actual steam turbines as a result of irreversibilities. In an effort to control entropy increase, it is proposed to cool the steam in the turbine by running cooling water around the turbine casing. It is argued that this will reduce the entropy and the enthalpy of the steam at the turbine exit and thus increase the work output.
How would you evaluate this proposal?

Answers: 2


Another question on Physics

Physics, 22.06.2019 08:00
5g of ammonium nitrate was dissolved in 60g of water in an insulated container. the temperature at the start of the reaction was 23.0°c and at the end it was 19.0°c. calculate the energy absorbed by the reaction.
Answers: 3


Physics, 22.06.2019 23:30
Macy always thought there were only a few hair colors blonde brown and black however when she actually began looking around she saw varying shades of these hair color what is a possible reason for so many different hair colors
Answers: 1

Physics, 23.06.2019 01:00
Which pair of quantities includes one quantity that increases as the other decreases during simple harmonic motion?
Answers: 2
You know the right answer?
The entropy of steam increases in actual steam turbines as a result of irreversibilities. In an effo...
Questions


Mathematics, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30


Social Studies, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30

Computers and Technology, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30

Biology, 30.08.2019 04:30

English, 30.08.2019 04:30




Social Studies, 30.08.2019 04:30






History, 30.08.2019 04:30


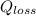 is the heat loss from the turbinee due to the cooking water.
is the heat loss from the turbinee due to the cooking water.

