Please help!
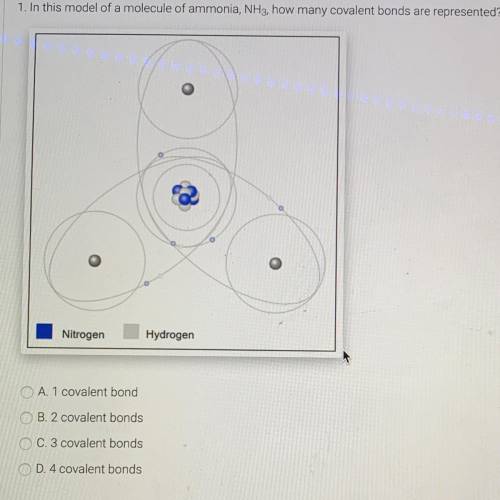
1. In this model of a molecule of ammonia, NH3, how many covalent bonds are repr...


Answers: 1


Another question on Physics


Physics, 22.06.2019 03:00
An internally reversible refrigerator has a modified coefficient of performance accounting for realistic heat transfer processes of where qin is the refrigerator cooling rate, qout is the heat rejection rate, and is the power input. show that copm can be expressed in terms of the reservoir temperatures tc and th, the cold and hot thermal resistances rt,c and rt,h, and qin, as where rtot rt,c rt,h. also, show that the power input may be expressed as 1.39 a household refrigerator operates with cold- and hot-temperature reservoirs of tc 5 c and th 25 c, respectively. when new, the cold and hot side resistances are rc,n 0.05 k/w and rh,n 0.04 k/w, respectively. over time, dust accumulates on the refrigerator’s condenser coil, which is located behind the refrigerator, increasing the hot side resistance to rh,d 0.1 k/w. it is desired to have a refrigerator cooling rate of qin 750 w. using the results of problem 1.38, determine the modified coefficient of performance and the required power input w under (a) clean and (b) dusty coil conditions. internally reversible refrigerator qout qin w high-temperature reservoir low-temperature reservoir th th,i tc,i tc high-temperature side resistance low-temperature side resistance w qin th tc qinrtot tc qinrtot copm tc qinrtot th tc
Answers: 2

Physics, 22.06.2019 07:30
Which of the following is an example of motion in two dimensions?
Answers: 3

Physics, 22.06.2019 10:00
Because air contracts as it cools, the air pressure inside a freezer is typically lower than on the outside. why do ice cubes inside a freezer tend to shrink over time? a. the ice dissolves oxygen from the air, forming a denser crystalline matrix.b. the ice reacts chemically with carbon dioxide in the air, forming gaseous carbon compounds.c. the ice melts, and then the liquid freezes as ice crystals on the bottom of the freezer.d. the ice sublimes, and then the water vapor deposits as ice crystals on the sides of the freezer.
Answers: 1
You know the right answer?
Questions










Mathematics, 13.01.2021 14:00

Chemistry, 13.01.2021 14:00