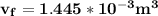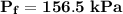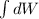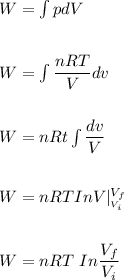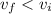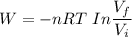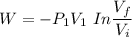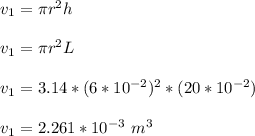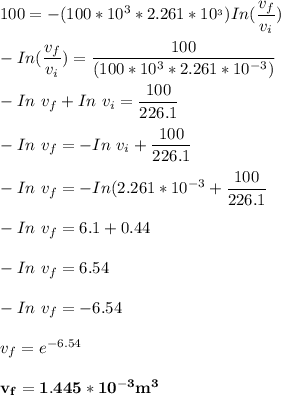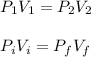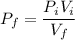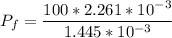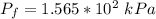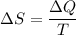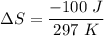Physics, 01.07.2020 15:01 Kekkdkskdkdk
A 12 cm diameter piston-cylinder device contains air at a pressure of 100 kPa at 24oC. The piston is initially 20 cm from the base of the cylinder. The gas is now compressed and 0.1 kJ of boundary work is added to the gas. The temperature of the gas remains constant during this process.
a. How much heat was transferred to/from the gas?
b. What is the final volume and pressure in the cylinder?
c. Find the change in entropy of the gas. Why is this value negative if entropy always increases in actual processes?

Answers: 2


Another question on Physics

Physics, 21.06.2019 20:10
Which force is most responsible for binding together an atom's protons and neutrons? electrostatic gravitational nuclear magnetic
Answers: 1


Physics, 22.06.2019 19:30
Aplayground slide is 8.80 ft long and makes an angle of 25.0° with the horizontal. a 63.0-kg child, initially at the top, slides all the way down to the bottom of the slide. (a) choosing the bottom of the slide as the reference configuration, what is the system's potential energy when the child is at the top and at the bottom of the slide? what is the change in potential energy as the child slides from the top to the bottom of the slide? (include the sign of the value in your answer.)
Answers: 3

Physics, 22.06.2019 21:00
Earth's atmosphere is composed of mostly two gases. name the two gases that make up the majority of the atmosphere and use the pie chart to tell the percentages of each.
Answers: 2
You know the right answer?
A 12 cm diameter piston-cylinder device contains air at a pressure of 100 kPa at 24oC. The piston is...
Questions




Biology, 13.11.2019 22:31


Mathematics, 13.11.2019 22:31

History, 13.11.2019 22:31




Physics, 13.11.2019 22:31

Chemistry, 13.11.2019 22:31