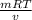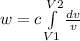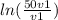Answers: 2


Another question on Physics

Physics, 22.06.2019 01:20
Un jugador de beisbol batea un foul recto en el aire.la pelota deja el bate con una rapidez de 120 km/h. en ausencia de resistencia del aire. ? cual sera la rapidez de la pelota cuando la atrape el catcher
Answers: 1

Physics, 22.06.2019 01:30
Kate is working on a project in her tech education class. she plans to assemble a fan motor. which form of energy does the motor convert most of its electric energy into? a. light energy b. motion energy c. sound energy d. thermal energy
Answers: 1

Physics, 22.06.2019 07:50
Imagine you are standing in the antarctic during the southern hemisphere summer. the sky is clear and blue. there are no clouds in the sky. your shadow is cast on the icy ground. what is the color of the shadow?
Answers: 1

Physics, 22.06.2019 09:50
A250-n box is at rest on a frictionless horizotal floor. then a person shoves the box with a non-zero force of magnitude p. which statement best describes the relationship betweeen p and the motion of the box after it was shoved? -the box will not move when p< 250n but otherwise will accelerate at a constant rate that is directly proportional to p - the box will begin moving at a speed that is directly proportional to p, then will gradually slow to a halt -the box will move with constant velocity at a speed that is directly proportional to p -box will accelerate across the floor at a constant rate that is directly proportional to p
Answers: 1
You know the right answer?
An ideal gas in a balloon is kept in thermal equilibrium with its constant-temperature surroundings....
Questions



Mathematics, 27.04.2021 16:00

Mathematics, 27.04.2021 16:00

English, 27.04.2021 16:00

Mathematics, 27.04.2021 16:00






Geography, 27.04.2021 16:00



Geography, 27.04.2021 16:00



History, 27.04.2021 16:00