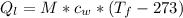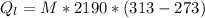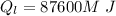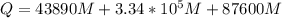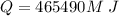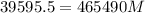Physics, 15.07.2020 04:01 maryjane8872
An insulated beaker with negligible mass contains a mass of 0.300 kgkg of water at a temperature of 71.5°C. Take the specific heat for water to be 4190 J/kg⋅K, the specific heat for ice to be 2100 J/kg⋅K, and the heat of fusion for water to be 334 kJ/kgJ.
Required:
How much ice at a temperature of -20.9 ∘C must be dropped into the water so that the final temperature of the system will be 40.0°C ?

Answers: 2


Another question on Physics

Physics, 22.06.2019 14:30
What distance does a car travel as its speed changes from 0 to 20 m/s in 17 s at constant acceleration?
Answers: 1


Physics, 22.06.2019 16:00
Apersons beliefs and general outlook, which act like filters on the information they receive are called ?
Answers: 1

Physics, 22.06.2019 16:30
In a hydrogen molecule there are a total of four charges, 2 protons in the two nuclei, and 2 electrons. how many unique charge-pairs are there (without double counting)?
Answers: 3
You know the right answer?
An insulated beaker with negligible mass contains a mass of 0.300 kgkg of water at a temperature of...
Questions

Biology, 04.02.2021 05:00


History, 04.02.2021 05:00

Mathematics, 04.02.2021 05:00


Chemistry, 04.02.2021 05:00

History, 04.02.2021 05:00

English, 04.02.2021 05:00



Mathematics, 04.02.2021 05:00

Social Studies, 04.02.2021 05:00

English, 04.02.2021 05:00

Mathematics, 04.02.2021 05:00


Biology, 04.02.2021 05:00

History, 04.02.2021 05:00


History, 04.02.2021 05:00

Advanced Placement (AP), 04.02.2021 05:00

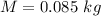
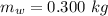
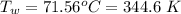
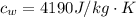
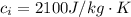
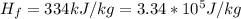
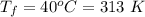
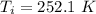
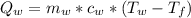
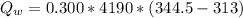
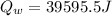
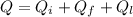
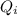 is the amount of heat absorbed by the ice to get to
is the amount of heat absorbed by the ice to get to 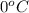 (273 K)and this is mathematically represented as
(273 K)and this is mathematically represented as 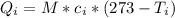
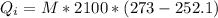
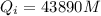
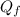 is the heat gained by the ice as it is been converted to liquid
is the heat gained by the ice as it is been converted to liquid 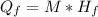
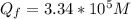
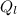 is the heat gained by the ice(now water) as it is been heated to
is the heat gained by the ice(now water) as it is been heated to 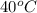 and it is mathematically represented as
and it is mathematically represented as