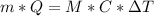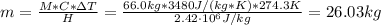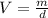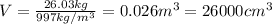The evaporation of sweat is an important mechanism for temperature control in some warm-blooded animals.
a. What mass of water must evaporate from the skin of a 66.0 kg man to cool his body 1.30 °C? The heat of vaporization of water at body temperature (37.0 ∘C) is 2.42×10^6J/kg. The specific heat capacity of a typical human body is 3480 J/(kg⋅K).
b. What volume of water must the man drink to replenish the evaporated water? Compare this result with the volume of a soft-drink can, which is 355 cm^3

Answers: 2


Another question on Physics

Physics, 22.06.2019 06:30
=force × distance a. work b. velocity c. pressure d. momentum
Answers: 1

Physics, 22.06.2019 10:20
Asmall object with mass 0.200 kg swings back and forth on the lower end of a light rope that is 3.00 m long. the upper end of the rope is attached to the ceiling. as the object swings through its lowest position, where the rope is vertical, the speed of the object is 5.80 m/s. at this point in the motion, what is the tension in the rope? (use g = 9.80 m/s2.)
Answers: 2

Physics, 22.06.2019 11:30
Which of the following is the phase that results when the moon is on the opposite side of the earth from the sun? a. quarter moon b. crescent moon c. new moon d. full moon
Answers: 1

Physics, 22.06.2019 12:40
Estimate the schwarzschild radius (in kilometers) for a mini-black hole formed when a superadvanced civilization decides to punish you (unfairly) by squeezing you until you become so small that you disappear inside your own event horizon. (assume that the your weight is 50 kg)
Answers: 1
You know the right answer?
The evaporation of sweat is an important mechanism for temperature control in some warm-blooded anim...
Questions




Arts, 29.11.2021 20:00


Mathematics, 29.11.2021 20:00

Biology, 29.11.2021 20:00

Mathematics, 29.11.2021 20:00


English, 29.11.2021 20:00

Mathematics, 29.11.2021 20:00




Geography, 29.11.2021 20:00





Mathematics, 29.11.2021 20:00