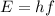Physics, 12.08.2020 04:01 angelinamadriga
A laser emits photons having an energy of 3.74 × 10–19 J. What color would be expected for the light emitted by this laser? (c = 3.00 × 108 m/s, h = 6.63 × 10–34 J ⋅ s)

Answers: 1


Another question on Physics

Physics, 22.06.2019 14:10
Click the game tab at the bottom of the simulation and select level 1. (there is no seesaw balance for this part of the activity.) balance the first equation, and click check to see if you got it right. if you can’t balance it in the first try, you can try again. work through the five equations for level 1. click continue to go on to level 2, and later level 3. each level is more difficult than the one before. keep trying until all the equations are balanced. in one or two sentences, describe how you did in the balancing game. in a few more sentences, explain one strategy you learned for balancing more complex equations.
Answers: 2

Physics, 23.06.2019 00:30
Madison lives near the ocean. she’s formed a hypothesis that increased concentrations of salt in the air speeds the corrosion of certain metals. if madison plans to test this hypothesis, she will have to deal with the following variables in her experiment: dependent variable: independent variable: one possible confounding variable:
Answers: 1

Physics, 23.06.2019 02:00
Calculate the numbers of photons having a wavelength of 10.0 mm required to produce 1.0 kj of energycalculate the numbers of photons having a wavelength of 10.0 mm required to produce 1.0 kj of energy
Answers: 1

You know the right answer?
A laser emits photons having an energy of 3.74 × 10–19 J. What color would be expected for the light...
Questions

Computers and Technology, 03.12.2021 01:00


Chemistry, 03.12.2021 01:00

Computers and Technology, 03.12.2021 01:00



History, 03.12.2021 01:00


English, 03.12.2021 01:00

Mathematics, 03.12.2021 01:00

Computers and Technology, 03.12.2021 01:00

Social Studies, 03.12.2021 01:00

Mathematics, 03.12.2021 01:00

Social Studies, 03.12.2021 01:00



Mathematics, 03.12.2021 01:00


Social Studies, 03.12.2021 01:00