
Physics, 13.08.2020 18:01 KekePonds1021
In state-of-the-art vacuum systems, pressures as low as 1.00 10-9 Pa are being attained. Calculate the number of molecules in a 1.90-m3 vessel at this pressure and a temperature of 28.0°C. molecules

Answers: 2


Another question on Physics

Physics, 22.06.2019 05:50
There are two routes similar in terrain; however, one route has an incline of approximately 28 degrees, and you must decide on which route to take. what is the maximum road grade that your fully loaded ecv can climb?
Answers: 2

Physics, 22.06.2019 11:30
If we relied solely on the nonrenewable resources found in the u.s., which one would we run out of first at current usage levels? a. natural gas b. oil c. uranium d. coal
Answers: 1

Physics, 22.06.2019 13:30
Select the three ways that the parallel-plate capacitor differs from a car battery.
Answers: 1

Physics, 22.06.2019 19:30
Ashot putter releases the shot some distance above the level ground with a velocity of 12.0 m/s, 51.0 ∘above the horizontal. the shot hits the ground 2.08 s later. you can ignore air resistance. how far did she throw the shot?
Answers: 2
You know the right answer?
In state-of-the-art vacuum systems, pressures as low as 1.00 10-9 Pa are being attained. Calculate t...
Questions


Mathematics, 22.07.2019 16:00

History, 22.07.2019 16:00


Social Studies, 22.07.2019 16:00

History, 22.07.2019 16:00


Chemistry, 22.07.2019 16:00



Mathematics, 22.07.2019 16:00



Biology, 22.07.2019 16:00


Mathematics, 22.07.2019 16:00


Mathematics, 22.07.2019 16:00


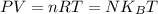
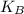 is Boltzmann's constant, = 1.38 x 10⁻²³ J/K
is Boltzmann's constant, = 1.38 x 10⁻²³ J/K

