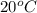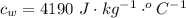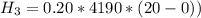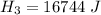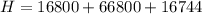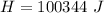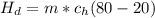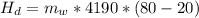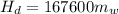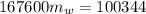In a container of negligible mass, 020 kg of ice at an initial temperature of - 40.0 oC is mixed with a mass m of water that has an initial temperature of 80.0 oC. No heat is lost to the surroundings. If the final temperature of the system is 20.0 oC, what is the mass m of the water that was initially at 80.0 oC

Answers: 2


Another question on Physics

Physics, 21.06.2019 22:00
Explain what makes a passenger in a turning car slide toward the door. critical thinking
Answers: 1

Physics, 22.06.2019 10:00
The frequencies refer to the sample data collected from a population of interest when performing a hypothesis test comparing two or more population proportions.
Answers: 2

Physics, 22.06.2019 13:00
Nacidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused the anode to lose 0.584 g after 1.52 ✕ 103 s. given that the charge of an electron is 1.6022 ✕ 10−19 c, calculate avogadro's number. assume that copper is oxidized to cu2+ ions.
Answers: 1

Physics, 22.06.2019 15:50
Ryan is examining the energy of the particles in a bar of gold. what is ryan most likely studying?
Answers: 1
You know the right answer?
In a container of negligible mass, 020 kg of ice at an initial temperature of - 40.0 oC is mixed wit...
Questions

Mathematics, 21.03.2020 05:18

Mathematics, 21.03.2020 05:18



Biology, 21.03.2020 05:19

Chemistry, 21.03.2020 05:19

Mathematics, 21.03.2020 05:19

Mathematics, 21.03.2020 05:19

Mathematics, 21.03.2020 05:19


Health, 21.03.2020 05:20


Mathematics, 21.03.2020 05:20



Mathematics, 21.03.2020 05:20

Mathematics, 21.03.2020 05:20

Biology, 21.03.2020 05:20

Mathematics, 21.03.2020 05:20

Mathematics, 21.03.2020 05:21

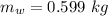
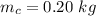
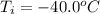
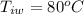
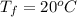
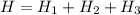
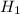 is the energy required to move the ice from
is the energy required to move the ice from 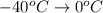

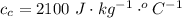
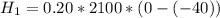
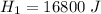
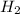 is the energy to melt the ice
is the energy to melt the ice 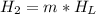
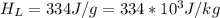
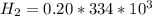
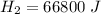
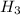 is the energy to raise the melted ice to
is the energy to raise the melted ice to