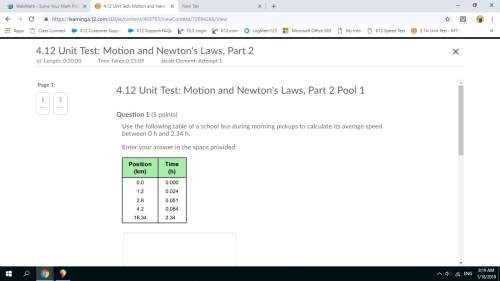
1401S
56. Steam at 100°C, is passed into a container of negligible heat capacity, containing
2...

Physics, 28.08.2020 05:01 coolman5999alt
1401S
56. Steam at 100°C, is passed into a container of negligible heat capacity, containing
20g of ice and 100g of water at 0°C, until the ice is completely melted. Determine the
total mass of water in the container. [Specific latent heat of steam = 2300Jg
specific latent heat of ice = 340 specific heat capacity of water = 4.2 Jg 'K'l

Answers: 2


Another question on Physics

Physics, 21.06.2019 20:00
Player kicks a soccer ball from ground level and sends it flying at an angle of 30 degrees at a speed of 26 m/s. what is the horizontal velocity component of the ball to the nearest tenth of a m/s?
Answers: 2

Physics, 22.06.2019 01:00
Complete the sentence to describe the law of conservation of energy. the law of conservation of energy states that energy cannot be created or , but it can be
Answers: 1

Physics, 22.06.2019 18:00
Which statement is the best definition of an atom? a) anything that has mass and occupies space. b) the smallest particle that has the properties of an element. c) a substance that cannot be broken down chemically into simpler substances. eliminate d) the smallest unit of a substance that exhibits all of the properties characteristic of that substance.
Answers: 2

Physics, 22.06.2019 18:30
Examples of states of consciousness include a. daydreaming b. dreaming during sleep c. hypnosis d. all of the above
Answers: 2
You know the right answer?
Questions

Mathematics, 25.08.2019 03:30


Chemistry, 25.08.2019 03:30




Mathematics, 25.08.2019 03:30


History, 25.08.2019 03:30

History, 25.08.2019 03:30





English, 25.08.2019 03:30

Mathematics, 25.08.2019 03:30

History, 25.08.2019 03:50

Biology, 25.08.2019 03:50