
Physics, 08.10.2020 01:01 ashtyndowdle
2. The visible region of the hydrogen spectrum results from relaxation of electrons from excited states to energy level 2 (n1). Use the Rydberg equation and your measured wavelengths to determine the energy transitions associated with each of your observed wavelengths for hydrogen. In other words, calculate the excited state energy level (n2) for each of your observed wavelengths for hydrogen. n has integer values; so, calculate it first with appropriate significant digits, then round it to an integer. Use the key to show your work for at least one calculation. Must show energy levels for each hydrogen wavelength.

Answers: 2


Another question on Physics


Physics, 22.06.2019 04:30
Work out sian speed for the first 30 minutes of her journey. give your answer in km/h.
Answers: 1

Physics, 22.06.2019 06:10
Which transition by an electron will release the greatest amount of energy? oa ob oc od
Answers: 2

Physics, 22.06.2019 19:50
Ahuge (essentially infinite) horizontal nonconducting sheet 10.0 cm thick has charge uniformly spread over both faces. the upper face carries +95.0 nc/m2 while the lower face carries -25.0 nc/ m2. what is the magnitude of the electric field at a point within the sheet 2.00 cm below the upper face? (ε0 = 8.85 × 10-12 c2/n · m2)
Answers: 1
You know the right answer?
2. The visible region of the hydrogen spectrum results from relaxation of electrons from excited sta...
Questions



History, 03.12.2021 01:00


Physics, 03.12.2021 01:00


Social Studies, 03.12.2021 01:00



Mathematics, 03.12.2021 01:00

Computers and Technology, 03.12.2021 01:00

Computers and Technology, 03.12.2021 01:00


Arts, 03.12.2021 01:00

Physics, 03.12.2021 01:00

Chemistry, 03.12.2021 01:00




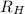 (1/2² - 1 / n²)
(1/2² - 1 / n²)


