
Physics, 15.10.2020 09:01 Onlyoneeniyaaa
A large research balloon containing 2.00 × 10^3 m^3 of helium gas at 1.00 atm and a temperature of 15.0°C rises rapidly from ground level to an altitude at which the atmospheric pressure is only 0.900 atm. Assume the helium behaves like an ideal gas and the balloon’s ascent is too rapid to permit much heat exchange with the surrounding air.
Required:
a. Calculate the volume of the gas at the higher altitude.
b. Calculate the temperature of the gas at the higher altitude.
c. What is the change in internal energy of the helium as the balloon rises to the higher altitude?

Answers: 3


Another question on Physics

Physics, 21.06.2019 22:00
If the speed of a particle triples ,by what factor does its kinetic energy increase?
Answers: 2


Physics, 23.06.2019 05:00
Which of the following is a transformer used for? alternate a voltage produce a voltage increase or decrease a voltage eliminate a voltage
Answers: 1

Physics, 23.06.2019 07:00
Atrain flies at a speed of 350 miles per hour how far will it travel in 3 hours
Answers: 2
You know the right answer?
A large research balloon containing 2.00 × 10^3 m^3 of helium gas at 1.00 atm and a temperature of 1...
Questions

Mathematics, 14.09.2020 15:01

Biology, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

History, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

History, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

English, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

English, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

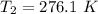
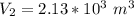
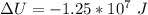
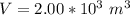
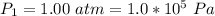
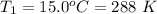
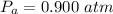
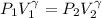
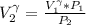
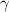 is a constant with value
is a constant with value 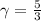 for an ideal gas
for an ideal gas 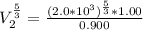
![V_2 = (\sqrt[5]{103.14641852} )^3](/tpl/images/0807/0306/7603b.png)
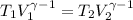
![T_2 = 288 * [\frac{2 * 10^{3}}{ 2.13 *10^{3}} ]^{ \frac{5}{3} -1 }](/tpl/images/0807/0306/ce7bb.png)
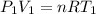
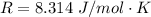
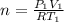

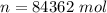
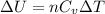
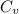 is the specific heats of gas at constant volume and the value is
is the specific heats of gas at constant volume and the value is 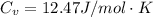
![\Delta U = 84362 * 12.47 * [T_2 - T_1 ]](/tpl/images/0807/0306/1f924.png)
![\Delta U = 84362 * 12.47 * [276.1 - 288 ]](/tpl/images/0807/0306/0b18f.png)


