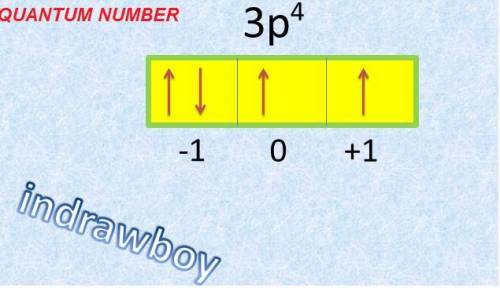
Which statement about electrons and atomic orbitals is not true?
an electron has the same amount of energy in all orbitals.
an orbital can contain a maximum of two electrons.
an electron cloud represents all the orbitals in an atom.
an atom’s lowest energy level has only one orbital.

Answers: 3


Another question on Physics

Physics, 22.06.2019 15:00
10 points! will mark brainiest! in a heat engine if 1,000 j of heat enters the system and the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2,000 j 1: write the equation2: list out your known variables 3: plug the numbers into the equations 4: solve 5: write your solution statement that includes initial energy and final energy added you so much!
Answers: 2


Physics, 22.06.2019 19:30
Refrigerant-134a flows through a carnot heat pump cycle at 0.5 kg/s. it is known that the maximum absolute temperature in the cycle is 1.1 times the minimum absolute temperature, and the net power input to the cycle is 2.5 kw. if the refrigerant changes from a saturated vapor to a saturated liquid during the heat rejection process, determine the maximum magnitude of the enthalpy of vaporization for this process in kj/kg (with 3 significant figures).
Answers: 3

Physics, 22.06.2019 19:30
The ability to make things happen is also called a. heat b. force c. matter d. energy
Answers: 2
You know the right answer?
Which statement about electrons and atomic orbitals is not true?
an electron has the same am...
an electron has the same am...
Questions

Mathematics, 03.12.2020 01:50

Advanced Placement (AP), 03.12.2020 01:50

Physics, 03.12.2020 01:50


Spanish, 03.12.2020 01:50




History, 03.12.2020 01:50


Mathematics, 03.12.2020 01:50




English, 03.12.2020 01:50

English, 03.12.2020 01:50



Health, 03.12.2020 01:50

History, 03.12.2020 01:50