
Physics, 17.10.2020 14:01 987654321156
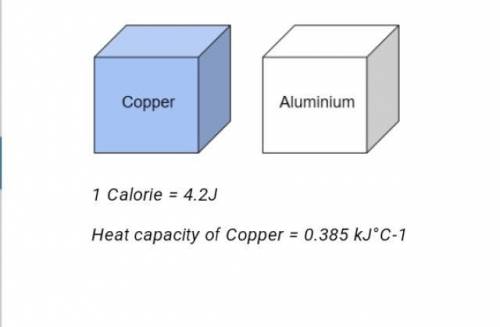
Two different blocks of metal are shown in the figure above. The copper block was heated from the temperature of 20°C to 120J°C, then brought in contact with the aluminum block. After some time, both metal blocks are measured at an equal temperature of 50°C. Based on the principle of calorimetry, find the number of calories transferred from the copper block to the aluminum block. 1 Calorie = 4.2J Heat capacity of Copper = 0.385 kJ°C-1

Answers: 1


Another question on Physics

Physics, 22.06.2019 09:00
Chemical energy is a form of energy. a. heat b. kinetic c. potential d. electromagnetic
Answers: 2

Physics, 22.06.2019 09:40
Asatellite component is in the shape of a cube with 10cm edges, has an evenly distributed mass of 2 kg and one bolt hole at each of four corners. if the maximum predicted random vibration environment is 10 grms, what bolt size would be a good choice for restraint? what other factors might drive bolt size besides force/stress?
Answers: 2


Physics, 23.06.2019 05:10
Which of the following should not be included in a great summary? a. blaming your lab partner b. new questions your experiment has generated c. your prediction d. your investigation plan
Answers: 2
You know the right answer?
Two different blocks of metal are shown in the figure above. The copper block was heated from the te...
Questions

English, 02.11.2020 14:00

English, 02.11.2020 14:00




Chemistry, 02.11.2020 14:00



History, 02.11.2020 14:00

English, 02.11.2020 14:00


Mathematics, 02.11.2020 14:00

English, 02.11.2020 14:00



Biology, 02.11.2020 14:00




Computers and Technology, 02.11.2020 14:00



