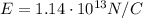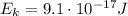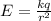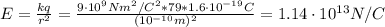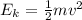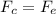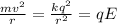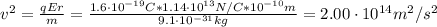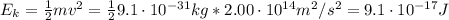Physics, 19.10.2020 20:01 Carrchris021
Before the development of quantum theory, Ernest Rutherford's experiments with gold atoms led him to propose the so-called Rutherford Model of atomic structure. The basic idea is that the nucleus of the atom is a very dense concentration of positive charge, and that negatively charged electrons orbit the nucleus in much the same manner as planets orbit a star. His experiments appeared to show that the average radius of an electron orbit around the gold nucleus must be about 10−1010−10 m. Stable gold has 79 protons and 118 neutrons in its nucleus.
What is the strength of the nucleus' electric field at the orbital radius of the electrons?
What is the kinetic energy of an electron in a circular orbit around the gold nucleus?

Answers: 1


Another question on Physics

Physics, 21.06.2019 18:00
Which surface feature of the moon is characterized by mountainous areas? terrae craters maria regolith
Answers: 1

Physics, 21.06.2019 19:00
What term do psychologists use to designate our personal awareness of feelings, sensations, and thoughts?
Answers: 1

Physics, 21.06.2019 20:00
Which of the following represents an upright image? a. -do b. +m c. -m d. +do
Answers: 1

Physics, 21.06.2019 23:00
Atrain departs from its station at a constant acceleration of 5 m/s. what is the speed of the train at the end of 20s?
Answers: 1
You know the right answer?
Before the development of quantum theory, Ernest Rutherford's experiments with gold atoms led him to...
Questions

Chemistry, 31.03.2020 05:42

Mathematics, 31.03.2020 05:42


Physics, 31.03.2020 05:42

Mathematics, 31.03.2020 05:42


Social Studies, 31.03.2020 05:43


Mathematics, 31.03.2020 05:43

Mathematics, 31.03.2020 05:43




Physics, 31.03.2020 05:43

Mathematics, 31.03.2020 05:43

Mathematics, 31.03.2020 05:43


Advanced Placement (AP), 31.03.2020 05:43


Mathematics, 31.03.2020 05:43