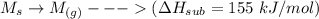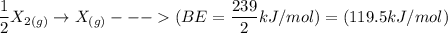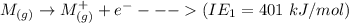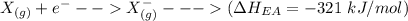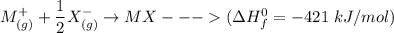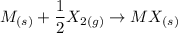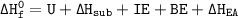Physics, 26.10.2020 17:00 10242000cw
Consider an ionic compound, MXMX , composed of generic metal MM and generic, gaseous halogen XX . The enthalpy of formation of MXMX is ΔH∘f=−421ΔHf∘=−421 kJ/mol. The enthalpy of sublimation of MM is ΔHsub=155ΔHsub=155 kJ/mol. The ionization energy of MM is IE=401IE=401 kJ/mol. The electron affinity of XX is ΔHEA=−321ΔHEA=−321 kJ/mol. (Refer to the hint). The bond energy of X2X2 is BE=239BE=239 kJ/mol. Determine the lattice energy of MXMX .

Answers: 2


Another question on Physics

Physics, 22.06.2019 00:00
An inclined plane wrapped around a metal shaft is a a. lever b. screw c. pulley d. wedge
Answers: 2

Physics, 22.06.2019 05:30
Aforklift raises a 1,020 n crate 3.50 m up to a shelf. how much work is done by the forklift on the crate? the forklift does j of work on the crate.
Answers: 2


Physics, 22.06.2019 10:40
As you are trying to move a heavy box of mass m, you realize that it is too heavy for you to lift by yourself. there is no one around to , so you attach an ideal pulley to the box and a massless rope to the ceiling, which you wrap around the pulley. you pull up on the rope to lift the box. use g for the magnitude of the acceleration due to gravity and neglect friction forces. once you have pulled hard enough to start the box moving upward, what is the magnitude f of the upward force you must apply to the rope to start raising the box with constant velocity? express the magnitude of the force in terms of m, the mass of the box.
Answers: 1
You know the right answer?
Consider an ionic compound, MXMX , composed of generic metal MM and generic, gaseous halogen XX . Th...
Questions


Mathematics, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01

History, 13.10.2020 15:01





History, 13.10.2020 15:01



Advanced Placement (AP), 13.10.2020 15:01


Mathematics, 13.10.2020 15:01





History, 13.10.2020 15:01

Arts, 13.10.2020 15:01

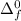 = - 421 kJ/mol
= - 421 kJ/mol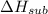 = 155 kJ/mol
= 155 kJ/mol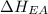 = -321 kJ/mol
= -321 kJ/mol