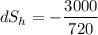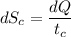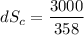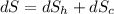When an aluminum bar is connected between a hot reservoir at 720 K and a cold reservoir at 358 K, 3.00 kJ of energy is transferred by heat from the hot reservoir to the cold reservoir. (a) In this irreversible process, calculate the change in entropy of the hot reservoir. J/K
(b) In this irreversible process, calculate the change in entropy of the cold reservoir.
J/K
(c) In this irreversible process, calculate the change in entropy of the Universe, neglecting any change in entropy of the aluminum rod.
J/K
(d) Mathematically, why did the result for the Universe in part (c) have to be positive?

Answers: 3


Another question on Physics


Physics, 22.06.2019 12:20
Which lists the pairs of plates in order from least to greatest in terms of the work done to move the electron?
Answers: 2

Physics, 23.06.2019 01:30
Mackinzie walks 4 blocks west, then 2 blocks south, then 4 blocks east, and,finally, 1 block south. what is the magnitude of her total displacement duringthis walk (in blocks)?
Answers: 2

Physics, 23.06.2019 01:30
Imagine that the rightward current flows in the rod for a short time. as a result, what will the net charge on the right and left ends of the rod become? left end negative and right end positive left end negative and right end negative left end negative and right end nearly neutral left end nearly neutral and right end positive both ends nearly neutral
Answers: 3
You know the right answer?
When an aluminum bar is connected between a hot reservoir at 720 K and a cold reservoir at 358 K, 3....
Questions




















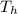 = 720 K
= 720 K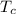 = 358 K
= 358 K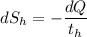 (the negative sign shows the loss of heat)
(the negative sign shows the loss of heat)