
Physics, 16.11.2020 16:30 middlegirlrule6848
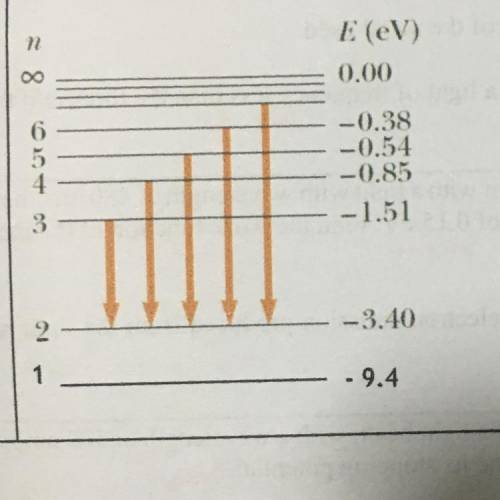
12. Figure below shows the energy levels for a hydrogen atom.
(a) Calculate the ionization energy for the atom.
(b) Calculate the frequency of the spectrum due to transition from n = 4 to n= 3.
Thank you!!

Answers: 2


Another question on Physics

Physics, 22.06.2019 04:50
Two technicians are discussing a resistance measurement between the can-h and can-l wires. technician a says this measurement should be done with the ignition switch in the "run" position. technician b states that a measurement of 0 ohms indicates an open in the network. which technician is correct?
Answers: 1

Physics, 22.06.2019 14:00
Me pl give an example of a collision in real life. use the law of conservation of energy to describe the transfer of momentum. be sure and discuss the momentum before and after the collision occurs. you will need at least 3 sentences to thoroughly answer this question.
Answers: 3

Physics, 22.06.2019 15:30
Charge is distributed along the entire x-axis with uniform density λ. how much work does the electric field of this charge distribution do on an electron that moves along the y-axis from y = a to y = b? (use the following as necessary: a, b, ε0, λ, and q for the charge on an electron.)
Answers: 3

Physics, 22.06.2019 16:30
Place several e-field sensors at a few points on different equipotential lines, and look at the relationship between the electric field and the equipotential lines. which statement is true? 1-at any point, the electric field is perpendicular to the equipotential line at that point, and it is directed toward lines of higher voltages. 2-at any point, the electric field is perpendicular to the equipotential line at that point, and it is directed toward lines of lower voltages. 3-at any point, the electric field is parallel to the equipotential line at that point.
Answers: 1
You know the right answer?
12. Figure below shows the energy levels for a hydrogen atom.
(a) Calculate the ionization energy f...
Questions

Mathematics, 15.12.2020 23:10

Mathematics, 15.12.2020 23:10

Mathematics, 15.12.2020 23:10

Mathematics, 15.12.2020 23:10





Mathematics, 15.12.2020 23:10





English, 15.12.2020 23:10








