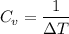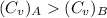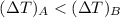Physics, 16.12.2020 16:30 geliyahmoore1
For constant volume processes the heat capacity of gas A is greater than the heat capacity of gas B. We conclude that when they both absorb the same energy as heat at constant volume:
A. the temperature of A increases more than the temperature of B
B. the temperature of B increases more than the temperature of A
C. the internal energy of A increases more than the internal energy of B
D. the internal energy of B increases more than the internal energy of A
E. A does more positive work than B

Answers: 1


Another question on Physics

Physics, 21.06.2019 18:20
Let f(x) = x4- 8x2 . find the relativeextrema of this function using the second derivative test.
Answers: 2

Physics, 21.06.2019 23:00
How many dots must be added to the symbol to properly represent a standard nitrogen ion? a) 1 b) 3 c) 5 d) 8
Answers: 1

Physics, 22.06.2019 09:40
When you jump from an elevated position you usually bend your knees upon reaching the ground. by doing this, you make the time of the impact about 10 times as great as for a stiff-legged landing. in this way the average force your body experiences is a) less than 1/10 as great. b) more than 1/10 as great. c) about 1/10 as great.d) about 10 times as great.
Answers: 1

You know the right answer?
For constant volume processes the heat capacity of gas A is greater than the heat capacity of gas B....
Questions


Mathematics, 27.01.2022 06:40

Mathematics, 27.01.2022 06:40





Mathematics, 27.01.2022 06:40



Physics, 27.01.2022 06:40

History, 27.01.2022 06:40


Mathematics, 27.01.2022 06:40


Advanced Placement (AP), 27.01.2022 06:40

Social Studies, 27.01.2022 06:40


Mathematics, 27.01.2022 06:50

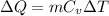
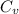 is heat capacity at constant volume
is heat capacity at constant volume