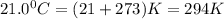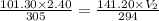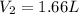Physics, 20.12.2020 21:20 singlegirlforlife541
At an ocean depth of 10.0m, a diver's lung capacity is 2.40L. The air temperature is 32.0°C and the pressure is 101.30 kPa. What is the volume of the diver's at the same depth, at a temperature of 21.0°C and a pressure of 141.20 kPa?

Answers: 2


Another question on Physics


Physics, 22.06.2019 16:40
On your wedding day your lover gives you a gold ring of mass 3.67 g. 48 years later its mass is 3.44 g. on the average how many atoms were abrated from the ring during each second of your marriage? the atomic mass of gold is 197 u. don't enter units
Answers: 1

Physics, 23.06.2019 00:00
Examine the drawing of a molecule. each ring represents an atom of nitrogen (n). what’s the chemical formula of this substance?
Answers: 2

Physics, 23.06.2019 00:30
Which of the following statements accurately describes the sign of the work done on the box by the force of the push? a. positive b. negative c. zero
Answers: 3
You know the right answer?
At an ocean depth of 10.0m, a diver's lung capacity is 2.40L. The air temperature is 32.0°C and the...
Questions







Mathematics, 21.02.2020 03:57

Mathematics, 21.02.2020 03:57

Mathematics, 21.02.2020 03:57




English, 21.02.2020 03:57

Mathematics, 21.02.2020 03:57

Advanced Placement (AP), 21.02.2020 03:57



Mathematics, 21.02.2020 03:57


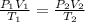
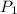 = initial pressure of gas = 101.30 kPa
= initial pressure of gas = 101.30 kPa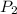 = final pressure of gas = 141.20 kPa
= final pressure of gas = 141.20 kPa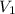 = initial volume of gas = 2.40 L
= initial volume of gas = 2.40 L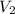 = final volume of gas = ?
= final volume of gas = ?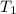 = initial temperature of gas =
= initial temperature of gas = 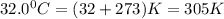
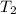 = final temperature of gas =
= final temperature of gas =