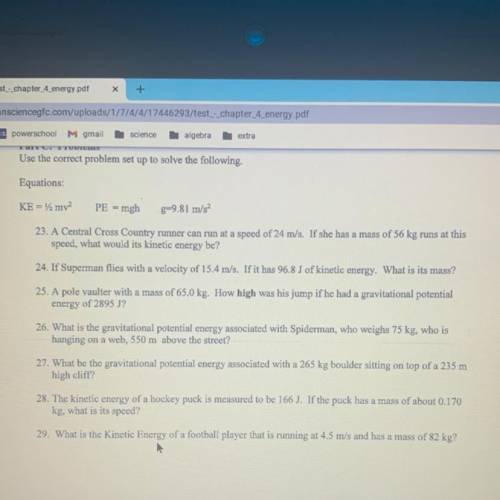
(need Ke=1/2mv2 & PE=mgh & g= 9.81 m/s2)
...

Answers: 2


Another question on Physics

Physics, 22.06.2019 05:50
Acylinder with a movable piston contains 11.7 moles of a monatomic ideal gas at a pressure of 1.32×10^5 pa. the gas is initially at a temperature of 300 k. an electric heater adds 43200 j of energy into the gas while the piston moves in such a way that the pressure remains constant. cp=20.79 j k^−1 mol^−1 for a monatomic ideal gas, and that the number of gas molecules is equal to avogadro's number (6.022×10^23) times the number of moles of the gas. (a) what is the temperature of the gas after the energy is added? (b) what is the change in volume of the gas? (c) how much work is done by the gas during this process?
Answers: 3

Physics, 22.06.2019 06:30
The mini-refrigerator fire was most likely caused by what type of wiring?
Answers: 2

Physics, 22.06.2019 09:30
Which are advantages of renewable resources? check all that apply. renewable energy supplies are completely reliable everywhere. some renewable resources will never be used up. little or no waste is produced by renewable resource plants. electricity can be generated in large quantities. many renewable energy facilities have lower operating costs.
Answers: 1

Physics, 22.06.2019 14:00
What is the force that opposes motion and works against the downward pull? a) friction b) gravity c) weight d) acceleration
Answers: 1
You know the right answer?
Questions

Mathematics, 16.10.2020 07:01





Mathematics, 16.10.2020 07:01


Mathematics, 16.10.2020 07:01

Mathematics, 16.10.2020 07:01





Mathematics, 16.10.2020 07:01


Geography, 16.10.2020 07:01