
Physics, 25.12.2020 14:00 tiffxnnyyy
Find the quantity of heat needed
to melt 100g of ice at -10 °C
into water at 10 °C. (39900 J)
(Note: Specific heat of ice is
2100 Jkg 'K', specific heat
of water is 4200 Jkg K',
Latent heat of fusion of ice is
336000 Jkg ').

Answers: 2


Another question on Physics

Physics, 21.06.2019 18:00
According to the law of conservation of mass, in a chemical reaction the total starting mass of all the reactants equal the total final mass of all the products. true or false?
Answers: 1

Physics, 21.06.2019 20:50
Aball is thrown upward in the air, and its height above the ground after t seconds is h ( t ) = 57 t − 16 t 2 feet. find the time t when the ball will be traveling upward at 14.25 feet per second.
Answers: 1

Physics, 22.06.2019 13:30
Global warming will produce rising sea levels partly due to melting ice caps but also due to the expansion of water as average ocean temperatures rise. to get some idea of the size of this effect, calculate the change in length of a column of water 1.00 km high for a temperature increase of 1.00ºc. note that this calculation is only approximate because ocean warming is not uniform with depth. (answer in ×10^{-3} −3 m)
Answers: 1

You know the right answer?
Find the quantity of heat needed
to melt 100g of ice at -10 °C
into water at 10 °C. (39900 J)<...
into water at 10 °C. (39900 J)<...
Questions

History, 09.12.2020 01:00


Mathematics, 09.12.2020 01:00




Mathematics, 09.12.2020 01:00




Business, 09.12.2020 01:00






Mathematics, 09.12.2020 01:00

Chemistry, 09.12.2020 01:00

Mathematics, 09.12.2020 01:00

Mathematics, 09.12.2020 01:00

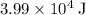 (assuming that the melting point of ice is
(assuming that the melting point of ice is 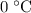 .)
.) 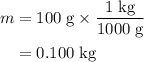
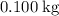 of ice from
of ice from 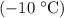 to
to 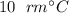 .
.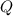 required to raise the temperature of a sample of mass
required to raise the temperature of a sample of mass 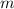 and specific heat capacity
and specific heat capacity  by
by  :
: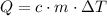 ,
, 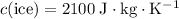 whereas
whereas 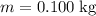 . Calculate the change in the temperature:
. Calculate the change in the temperature: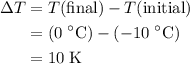 .
.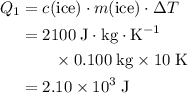 .
.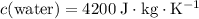 whereas
whereas 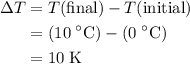 .
.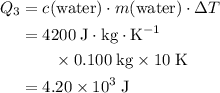 .
.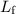 is:
is: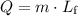 .
.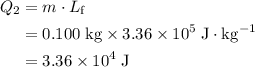 .
.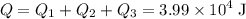 .
.

